Human gut commensal Alistipes timonensis modulates the host lipidome and delivers anti-inflammatory outer membrane vesicles to suppress colitis in an Il10-deficient mouse model
- PMID: 40497338
- PMCID: PMC12160598
- DOI: 10.1080/19490976.2025.2517380
Human gut commensal Alistipes timonensis modulates the host lipidome and delivers anti-inflammatory outer membrane vesicles to suppress colitis in an Il10-deficient mouse model
Abstract
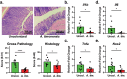
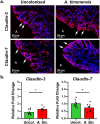
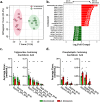
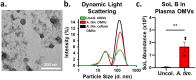
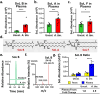
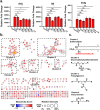
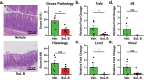
Correlative studies have linked human gut microbes to specific health conditions. Alistipes is one such microbial genus negatively linked to inflammatory bowel disease (IBD). However, the protective role of Alistipes in IBD is understudied, and the underlying molecular mechanisms remain unknown. In this study, colonization of Il10-deficient mice with Alistipes timonensis DSM 27924 delays colitis development. Colonization does not significantly alter the gut microbiome composition, but instead shifts the host plasma lipidome, increasing phosphatidic acids while decreasing triglycerides. Outer membrane vesicles (OMVs) derived from Alistipes are detected in the plasma of colonized mice, carrying potentially immunomodulatory metabolites into the host circulatory system. Fractions of A. timonensis OMVs suppress LPS-induced Il6, Il1b, and Tnfa expression in vitro in murine macrophages. We detect putative bioactive lipids in the OMVs, including immunomodulatory sulfonolipids (SoLs) in the active fraction, which are also increased in the blood of colonized mice. Treating Il10-deficient mice with purified SoL B, a representative SoL, suppresses colitis development, suggesting its contribution to the anti-inflammatory phenotype observed with A. timonensis colonization. Thus, A. timonensis OMVs represent a potential mechanism for Alistipes-mediated delay of colitis in Il10-deficient mice via delivery of immunomodulatory lipids and modulation of the host plasma lipidome.
Keywords: Alistipes; IBD; Il10-deficient mice; OMVs; colitis; gut microbes; inflammation; sulfonolipids.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Update of
-
Human gut commensal Alistipes timonensis modulates the host lipidome and delivers anti-inflammatory outer membrane vesicles to suppress colitis in an Il10 -deficient mouse model.bioRxiv [Preprint]. 2025 Jun 2:2024.10.23.619966. doi: 10.1101/2024.10.23.619966. bioRxiv. 2025. Update in: Gut Microbes. 2025 Dec;17(1):2517380. doi: 10.1080/19490976.2025.2517380. PMID: 39484420 Free PMC article. Updated. Preprint.
References
-
- FitzGerald R, Smith SM.. 2021. An overview of helicobacter pylori infection. In: Smith SM, editor. Helicobacter pylori. Vol. 2283. New York (NY): Springer; p. 1–22. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
