Mechanistic insights into dental stem cells-derived exosomes in regenerative endodontics
- PMID: 40497413
- PMCID: PMC12339802
- DOI: 10.1111/iej.14269
Mechanistic insights into dental stem cells-derived exosomes in regenerative endodontics
Abstract
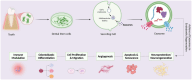
Background: Dental pulp is a richly vascularised and innervated tissue vital for tooth vitality, sensory function, and structural integrity. While conventional root canal therapy effectively treats necrotic permanent teeth, it irreversibly eliminates pulp vitality, potentially increasing the risk of secondary infections and long-term structural compromise. In response, regenerative endodontics has emerged as a biologically favourable alternative that seeks to restore the pulp-dentine complex using principles of tissue engineering.
Objectives: This review aims to explore the therapeutic potential and mechanisms of action of exosomes derived from dental stem cells (DSC-Exos), a subclass of mesenchymal stem cells (MSCs), in promoting regeneration of the pulp-dentine complex, while also addressing translational challenges and proposing an integrated regenerative framework.
Methods: A comprehensive literature search was conducted across Web of Science, PubMed, and Scopus databases using keywords associated with "stem cells," "exosomes," "extracellular vesicles," and "dental pulp regeneration." Titles and abstracts were screened, and eligible studies were selected based on predefined inclusion criteria: (a) original research or case reports focusing on DSC-Exos in regenerative endodontics, (b) in vitro and in vivo studies, and (c) clinical trials or animal studies showing pulp-like tissue development. Studies not fulfilling these criteria were excluded. A total of 67 articles were included for narrative synthesis.
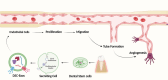
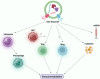
Results: DSC-Exos were found to facilitate multiple regenerative functions: promoting odontoblastic differentiation and dentine mineralisation, enhancing angiogenesis, regulating inflammation, modulating immune responses, promoting cell proliferation and migration, reducing apoptosis and senescence, and supporting neuroprotection. In-vivo studies demonstrated pulp-like tissue formation, revascularisation, and functional restoration. However, heterogeneity in exosome isolation, culture conditions, donor variability, and unclear molecular pathways remain unresolved issues.
Discussion: DSC-Exos present a promising acellular, immunologically safer approach to regenerative endodontics compared to direct stem cell transplantation. Despite their potential, the lack of standardised methodologies and incomplete understanding of their molecular interaction with odontoblasts hinders clinical translation. Integration of exosomes with scaffolds, growth factors, and endogenous cues may enhance regenerative efficacy.
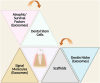
Conclusions: DSC-Exos represent a novel frontier in regenerative endodontics. This review proposes a triangular framework encompassing DSCs, exosomes, signalling molecules, scaffolds, and the dentine microenvironment to support a holistic and clinically translatable model for pulp-dentine complex regeneration.
Keywords: dental pulp; exosomes; regeneration; regenerative endodontics; stem cells.
© 2025 The Author(s). International Endodontic Journal published by John Wiley & Sons Ltd on behalf of British Endodontic Society.
Conflict of interest statement
All the authors declare that they have no conflicts of interest.
Figures

References
-
- Abdelgawad, L.M. , Nghnughi, M.H. & Abdelgwad, M. (2022) Influence of photo biomodulation using 980 nm diode laser and exsosomes derived from dental pulp stem cells on pulp regeneration of dogs' teeth. Reactions, 9(11), 4980–4985.
-
- Abdik, H. , Avsar Abdik, E. , Hızlı Deniz, A.A. , Taşlı, P.N. & Şahin, F. (2019) A novel virtue in stem cell research: exosomes and their role in differentiation. In: Turksen, K. (Ed.) Cell biology and translational medicine, volume 5. Advances in experimental medicine and biology. Cham: Springer, pp. 133–146. Available from: 10.1007/5584_2019_339 - DOI - PubMed

