Drebrin Upregulation Regulates Astrocyte Polarization and Supports Tissue Recovery After Spinal Cord Injury in Mice
- PMID: 40497424
- PMCID: PMC12313004
- DOI: 10.1002/glia.70048
Drebrin Upregulation Regulates Astrocyte Polarization and Supports Tissue Recovery After Spinal Cord Injury in Mice
Abstract
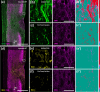
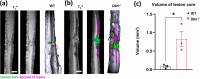
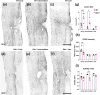
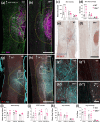
Spinal cord injury (SCI) results in significant disruption of nerve fibers responsible for transmitting signals between the brain and body, often leading to partial or complete motor, sensory, and autonomic dysfunction below the injury site. Astrocytes are an important component in scar formation, crucial for suppression of injury propagation, effective wound healing, and the regulation of neuronal plasticity. Here, we identify the role of the actin-binding protein Drebrin (DBN) in reactive astrogliosis following SCI. SCI induces the upregulation of DBN in astrocytes, which controls immediate injury containment but also the long-term preservation of tissue integrity and healing in the spinal cord. DBN knockout results in enlarged spinal cord lesions, increased immune cell infiltration, and neurodegeneration. Mechanistically, DBN loss disrupts the polarization of scar border-forming astrocytes, leading to impaired encapsulation of the injury. In summary, DBN serves as a pivotal regulator of SCI outcome by modulating astrocytic polarity, which is essential for establishing a protective barrier confining the lesion site.
Keywords: immune cell infiltration; neurodegeneration; reactive astrogliosis; spinal cord injury.
© 2025 The Author(s). GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Basso, D. M. , Fisher L. C., Anderson A. J., Jakeman L. B., Mctigue D. M., and Popovich P. G.. 2006. “Basso Mouse Scale for Locomotion Detects Differences in Recovery After Spinal Cord Injury in Five Common Mouse Strains.” Journal of Neurotrauma 23, no. 5: 635–659. 10.1089/neu.2006.23.635. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

