The human placenta and its role in reproductive outcomes revisited
- PMID: 40497429
- PMCID: PMC7617900
- DOI: 10.1152/physrev.00039.2024
The human placenta and its role in reproductive outcomes revisited
Abstract
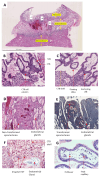
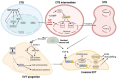
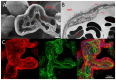
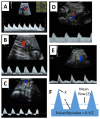
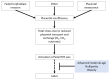
The placenta performs many key tasks that are essential for the healthy growth and development of the human fetus. Placental dysfunction has multiple manifestations, but they share the common property of lacking a mechanistic understanding of etiology. The clinical consequences of placental dysfunction are a major determinant of the global burden of disease. Currently, the primary clinical method for assessing placental function is ultrasonic Doppler flow velocimetry of the umbilical and uterine arteries. More recently, some biomarkers have emerged that can predict or diagnose placentally related complications of pregnancy. However, methods for identifying and characterizing placental dysfunction have developed relatively little over the last 20 years and perform poorly, and there remains an absence of disease-modifying therapies targeted at the placenta. Understanding disease mechanisms is made more difficult due to the profound differences in pregnancy and placentation comparing humans and the most commonly used laboratory animals, limiting the utility of animal models. The use of omics methods in human samples may yield progress: omics analyses of maternal blood show promise in identifying better predictors of disease, and single-cell analyses, including spatial omics of healthy and abnormal placentas, could identify therapeutic targets. Limitations in cellular models of the placenta have been significantly overcome in the last 5 to 10 years by the development of human cell models, including human trophoblast stem cells and organoids, and the use of these model systems may allow hypothesis testing experiments in a more clinically relevant context than animal models or immortalized cell lines.
Keywords: development; fetal growth restriction; preeclampsia; pregnancy; trophoblast.
Conflict of interest statement
The authors declare the following competing interests: GS and DC-J have received research support from Roche Diagnostics Ltd for studies of diagnostics and screening for fetal growth restriction and preeclampsia. GS’s department has received payment from Roche for a talk given by GS (fetal growth restriction). GS has been a paid consultant to GSK (preterm birth) and has been a member of a Data Monitoring Committee for GSK trials of RSV vaccination in pregnancy. GS is currently a member of a Data Monitoring Committee for RSV vaccination in pregnancy (Moderna) and chairs a Data Monitoring Committee for a hyperemesis gravidarum therapeutic trial (NG Biopharmaceuticals). GS and DC-J have received nonfinancial support from Illumina. GS and DC-J have received support from Pfizer and Sera Prognostics. Cambridge Enterprise (UK) have filed a patent relating to the prediction of pregnancy outcome with GS, DC-J and IA as named inventors. ST is a paid consultant to Diamedica Therapeutics and Avilar Therapeutics. He is on the scientific advisory board of Diamedica Therapeutics. ST is named on patents related to SPINT1 as a blood test for placental insufficiency and fetal growth restriction.
Figures




References
-
- Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, Abdollahpour I, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. - DOI - PMC - PubMed
-
- Smith GCS, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129 290 births. The Lancet. 2001;357:2002–2006. - PubMed
-
- Pell JP, Smith GCS, Walsh D. Pregnancy Complications and Subsequent Maternal Cerebrovascular Events: A Retrospective Cohort Study of 119,668 Births. Am J Epidemiol. 2004;159:336–342. - PubMed
-
- Poorthuis MHF, Algra AM, Algra A, Kappelle LJ, Klijn CJM. Female- and Male-Specific Risk Factors for Stroke: A Systematic Review and Meta-analysis. JAMA Neurol. 2017;74:75–81. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

