Amniotic fluid MSCs for scaffold-free cartilage repair: spheroid fusion and chondrogenic microtissue development
- PMID: 40497630
- PMCID: PMC12160606
- DOI: 10.1080/20565623.2025.2476922
Amniotic fluid MSCs for scaffold-free cartilage repair: spheroid fusion and chondrogenic microtissue development
Abstract
Background: Articular cartilage injuries are challenging due to limited regenerative capacity, causing chronic pain and impaired mobility. Current treatments are often inadequate, necessitating novel cartilage repair approaches. This study investigates amniotic fluid-derived mesenchymal stromal cells (AF-MSC) as a promising cell source for tissue engineering.
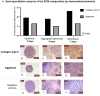
Research design and method: Cartilage-like microtissues were produced by differentiating AF-MSC into chondrocytes within a 3D culture system. Using a 3D-printed non-adhesive micromold, AF-MSC spheroids were formed and fused into larger microtissues. Spheroids were characterized for morphology, viability, and extracellular matrix (ECM) production. The mechanical properties of resulting microtissues were compared to native cartilage and agarose hydrogel.
Results: AF-MSC proved a viable, scalable cell source for cartilage microtissues. Spheroid fusion created structures with mechanical properties and ECM components resembling native cartilage.
Conclusions: AF-MSCs differentiated into chondrocytes when stimulated with TGF-β3 in a 3D micromolded culture, forming uniform, viable spheroids with robust ECM production and mechanical properties. These spheroids fused into neocartilage microtissue, showing potential for regenerative medicine, especially osteoarthritis treatment and drug testing. Further research should optimize conditions and evaluate long-term biomechanical performance.
Keywords: Mesenchymal stem cells; amniotic fluid; chondrogenesis; tissue engineering and cartilage regeneration.
Plain language summary
Cartilage injuries are hard to heal because cartilage does not repair itself easily. This can cause long-term pain and make movement difficult. Current treatments do not always work well, so scientists are looking for new ways to help cartilage heal. In this study, researchers used special cells from amniotic fluid, called mesenchymal stromal cells (AF-MSCs). These cells were grown in a 3D system, where they formed small, round clusters called spheroids. Over time, the spheroids merged to create tiny tissue structures similar to cartilage. The results showed that the cells stayed healthy, produced important cartilage materials, and had properties similar to natural cartilage. This method could help treat cartilage damage, including conditions like osteoarthritis. It could also be useful for testing new medicines. However, more research is needed to improve the process and make sure these engineered tissues remain stable over time.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures





References
References: Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers. : * The article provides a good background on regenerative medicine and cartilage injuries .
-
- Hunter DJ, Bierma-Zeinstra S.. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. - PubMed
*The article introduces the potential use of amniotic fluid mesenchymal cells in regenerative therapies.
-
- Prusa AR, Marton E, Rosner M, et al. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod. 2003;18(7):1489–1493. - PubMed
**The article provides a basic understanding of 3D cell culture for spheroid formation .
**These articles discuss the potential formation of viable microtissues resembling cartilage using mesenchymal cells .
LinkOut - more resources
Full Text Sources
