Application of a Multiomics Imaging Workflow to Explore Asparlas Treatment in Solid Tumors
- PMID: 40497651
- PMCID: PMC12199225
- DOI: 10.1021/acs.analchem.5c01503
Application of a Multiomics Imaging Workflow to Explore Asparlas Treatment in Solid Tumors
Abstract
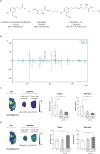
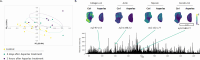
In acute lymphoblastic leukemia (ALL), hypermethylation of the asparagine synthetase (ASNS) gene promoter, leading to low levels of ASNS in tumor cells, is recognized as a prognostic biomarker, and l-asparaginase-based treatments (e.g., Asparlas) are frequently administered to these patients. In these cancers, tumor cells rely on external asparagine, and its depletion in the bloodstream results in tumor cell apoptosis. A multiomics (imaging) workflow is required to evaluate key molecular changes and characterize solid tumors to explore the potential efficacy of Asparlas in solid tumors. This study introduces a multiomics imaging workflow applicable to solid tumor specimens for the comprehensive molecular profiling of Asparlas treatment effects. The workflow integrates matrix-assisted laser desorption-ionization mass spectrometry imaging (MALDI-MSI), liquid chromatography coupled with high-resolution mass spectrometry, and histopathological staining on consecutive tumor tissue sections. It enables the detection and analysis of metabolites, lipids, and proteins. Tumor characterization was achieved through histology and clustering analysis based on lipid signatures, yielding consistent annotations. On-tissue chemical derivatization followed by MALDI-MSI was performed to assess metabolic alterations, with a focus on amino acids. ASNS distribution was mapped utilizing targeted MALDI-immunohistochemistry, followed by untargeted (spatial) proteomics on adjacent tissue sections. This study established a multiomics imaging approach and demonstrated its applicability in elucidating the metabolic changes in tumor tissue consequent to Asparlas treatment. Furthermore, it highlights the added value of multiomics imaging in pharmaceutical research and development.
Figures



Similar articles
-
Physical exercise training interventions for children and young adults during and after treatment for childhood cancer.Cochrane Database Syst Rev. 2016 Mar 31;3(3):CD008796. doi: 10.1002/14651858.CD008796.pub3. Cochrane Database Syst Rev. 2016. PMID: 27030386 Free PMC article.
-
PEG-asparaginase treatment regimens for acute lymphoblastic leukaemia in children: a network meta-analysis.Cochrane Database Syst Rev. 2023 May 31;5(5):CD014570. doi: 10.1002/14651858.CD014570.pub2. Cochrane Database Syst Rev. 2023. PMID: 37260073 Free PMC article.
-
Molecular feature-based classification of retroperitoneal liposarcoma: a prospective cohort study.Elife. 2025 May 23;14:RP100887. doi: 10.7554/eLife.100887. Elife. 2025. PMID: 40407808 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Diagnostic screening of COVID-19 based on multiomics data by high-resolution mass spectrometry (MALDI (+)-TOF MS and ESI(±)-Orbitrap MS).Metabolomics. 2025 Jun 29;21(4):94. doi: 10.1007/s11306-025-02299-z. Metabolomics. 2025. PMID: 40581904
References
-
- Zhang Y., Sultonova R. D., You S.-H., Choi Y., Kim S.-Y., Lee W.-S., Seong J., Min J.-J., Hong Y.. et al. The anticancer effect of PASylated calreticulin-targeting L-ASNase in solid tumor bearing mice with immunogenic cell death-inducing chemotherapy. Biochem. Pharmacol. 2023;210:115473. doi: 10.1016/j.bcp.2023.115473. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

