CCR2 recruits monocytes to the lung, while CX3CR1 modulates positioning of CD11cpos cells in the lymph node during pulmonary tuberculosis
- PMID: 40497732
- PMCID: PMC12239560
- DOI: 10.1128/mbio.01237-25
CCR2 recruits monocytes to the lung, while CX3CR1 modulates positioning of CD11cpos cells in the lymph node during pulmonary tuberculosis
Abstract
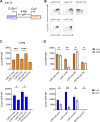
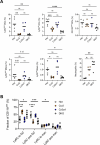
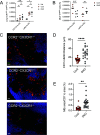
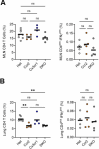
Infection by Mycobacterium tuberculosis (Mtb) continues to cause more than 1 million deaths annually, due to pathogen persistence in lung macrophages and dendritic cells derived from blood monocytes. While the accumulation of monocyte-derived cells in the Mtb-infected lung partially depends on the chemokine receptor CCR2, the other chemoattractant receptors regulating trafficking remain undefined. We used mice expressing knock-in/knockout reporter alleles of Ccr2 and Cx3cr1 to interrogate their expression and function in monocyte-derived populations of the lungs and draining mediastinal lymph nodes during Mtb infection. CCR2 and CX3CR1 expression varied across monocyte-derived subsets stratified by cell surface Ly6C expression in both organs. We found that the expression of CCR2 predicted the dependence of monocyte-derived cells on the receptor for lung and lymph node accumulation. CCR2-deficient mice were also observed to have worsened lung and lymph node Mtb burden. While CX3CR1 deficiency, alone or in combination with CCR2 deficiency, did not affect cell frequencies or lung Mtb control, its absence was associated with altered positioning of monocyte-derived dendritic cells in mediastinal lymph nodes. We found that the combined loss of Ccr2 and Cx3cr1 also worsened Mtb control in the mediastinal lymph node, suggesting a rationale for the persistent expression of CX3CR1 among monocyte-derived cells in pulmonary tuberculosis.IMPORTANCEMycobacterium tuberculosis (Mtb) is the respiratory pathogen responsible for the deadliest infectious disease worldwide. Susceptible humans exhibit ineffective immune responses, in which infected phagocytes are not able to eliminate the pathogen. Since recruited monocyte-derived cells serve as reservoirs for persistent infection, understanding how these phagocytes accumulate in the lung and why they are unable to eliminate Mtb can inform the development of therapies that can synergize with antimicrobials to achieve faster and more durable Mtb elimination. Monocyte-derived cells express the chemokine receptors CCR2 and CX3CR1, but the role of the latter in Mtb infection remains poorly defined. The significance of our study is in elucidating the roles of these receptors in the trafficking of monocyte-derived cells in the infected lung and mediastinal lymph node. These data shed light on the host response in tuberculosis and other pulmonary infections.
Keywords: Mycobacterium tuberculosis; cell trafficking; immunity; monocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Update of
-
CCR2 recruits monocytes to the lung, while CX3CR1 modulates positioning of monocyte-derived CD11c pos cells in the lymph node during pulmonary tuberculosis.bioRxiv [Preprint]. 2025 Feb 8:2025.02.07.637199. doi: 10.1101/2025.02.07.637199. bioRxiv. 2025. Update in: mBio. 2025 Jul 9;16(7):e0123725. doi: 10.1128/mbio.01237-25. PMID: 39974908 Free PMC article. Updated. Preprint.
References
-
- World Health Organization . 2024. Global tuberculosis report. World Health Organization, Geneva
MeSH terms
Substances
Grants and funding
- T32 HL007185/HL/NHLBI NIH HHS/United States
- F32 HL162424/HL/NHLBI NIH HHS/United States
- F31AI172360/National Institute of Allergy and Infectious Diseases
- T32 AI007180/AI/NIAID NIH HHS/United States
- T32 AI100853/AI/NIAID NIH HHS/United States
- P30 AI168440/AI/NIAID NIH HHS/United States
- R01 AI051242/AI/NIAID NIH HHS/United States
- R25 AI147375/AI/NIAID NIH HHS/United States
- U01 AI166309/AI/NIAID NIH HHS/United States
- F31 AI172360/AI/NIAID NIH HHS/United States
- P30 AI168440, R25 AI147375/National Institute of Allergy and Infectious Diseases
- T32 HL007185, F32 HL162424/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
