Functionalized Silver Nanoparticles as Multifunctional Agents Against Gut Microbiota Imbalance and Inflammation
- PMID: 40497864
- PMCID: PMC12157222
- DOI: 10.3390/nano15110815
Functionalized Silver Nanoparticles as Multifunctional Agents Against Gut Microbiota Imbalance and Inflammation
Abstract
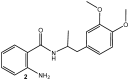
Human pathogenic fungi are the source of various illnesses, including invasive, cutaneous, and mucosal infections. One promising solution is using nanoparticles (NPs) as an antifungal agent. The current study aims to assess the antimicrobial and antifungal effects of drug-loaded silver nanoparticles (AgNPs) with previously reported mebeverine analogue (MA) as a potential drug candidate targeting gut microbiota and inflammation in the gastrointestinal tract. Density Functional Theory (DFT) calculations were conducted to identify possible mechanisms by which AgNPs could prevent microorganisms from growing. In vitro and ex vivo anti-inflammatory, in vitro antimicrobial, ex vivo spasmolytic activities, and in vitro hepatic cell morphology and proliferation of drug-loaded AgNPs were assessed. The drug-loaded AgNPs were considered to have promising antifungal activity against all tested fungal strains, Aspergillus niger, Penicillium chrysogenum, and Fusarium moniliforme, and yeasts, Candida albicans, Saccharomyces cerevisiae, and good antimicrobial activity against Gram-positive and Gram-negative bacterial strains. The results of in vitro and ex vivo determination of anti-inflammatory activity indicated that the drug-loaded AgNPs preserved MA's anti-inflammatory activity and decreased inflammation. A similar effect was observed in spasmolytic activity measurements. Drug-loaded AgNPs also influenced the morphology and proliferation of hepatic cells, indicating a potential for improved gut and liver therapeutic efficacy. Each test was performed in triplicate, and the results were reported as mean values. Based on the results, drug-loaded AgNPs might be a promising antimicrobial agent, maintaining the MA's potential as a spasmolytic and anti-inflammatory agent. Future in vivo and preclinical experiments will contribute to establishing the in vivo properties of drug-loaded AgNPs.
Keywords: DFT; anti-inflammatory activity; antifungal; antimicrobial; silver nanoparticles; spasmolytic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
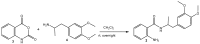






References
-
- Bajinka O., Darboe A., Tan Y., Abdelhalim K.A., Cham L.B. Gut Microbiota and the Human Gut Physiological Changes. Ann. Microbiol. 2020;70:65. doi: 10.1186/s13213-020-01608-2. - DOI
LinkOut - more resources
Full Text Sources

