Excellent Room-Temperature NO2 Gas-Sensing Properties of TiO2-SnO2 Composite Thin Films Under Light Activation
- PMID: 40497920
- PMCID: PMC12158011
- DOI: 10.3390/nano15110871
Excellent Room-Temperature NO2 Gas-Sensing Properties of TiO2-SnO2 Composite Thin Films Under Light Activation
Abstract
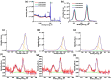
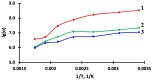
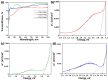
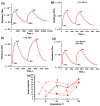
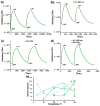
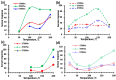
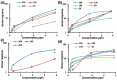
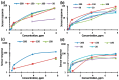
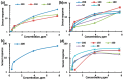
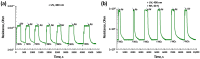
Thin TiO2-SnO2 nanocomposite films with high gas sensitivity to NO2 were synthesized by oxidative pyrolysis and comprehensively studied. The composite structure and quantitative composition of the obtained film nanomaterials have been confirmed by X-ray photoelectron spectroscopy, high-resolution transmission electron microscopy, and energy dispersive X-ray spectroscopy, which causes the presence of n-n heterojunctions and provides improved gas-sensitive properties. The sensor based on the 3TiO2-97SnO2 film has the maximum responses, which is explained by the existence of a strong surface electric field formed by large surface potentials in the region of TiO2-SnO2 heterojunctions detected by the Kelvin probe force microscopy method. Exposure to low-intensity radiation (no higher than 0.2 mW/cm2, radiation wavelength-400 nm) leads to a 30% increase in the sensor response relative to 7.7 ppm NO2 at an operating temperature of 200 °C and a humidity of 60% RH. At room temperature (20 °C), under humidity conditions, the response is 1.8 when exposed to 0.2 ppm NO2 and 85 when exposed to 7.7 ppm. The lower sensitivity limit is 0.2 ppm NO2. The temporal stability of the proposed sensors has been experimentally confirmed.
Keywords: SnO2; TiO2; composites; gas sensors; light activation; metal oxide; thin films.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Xu J., He X., Xu K., Liao H., Zhang C. Synthesis and Optimization Strategies of Nanostructured Metal Oxides for Chemiresistive Methanol Sensors. Ceram. Int. 2023;49:21113–21132. doi: 10.1016/j.ceramint.2023.03.274. - DOI
-
- Krishna K.G., Parne S., Pothukanuri N., Kathirvelu V., Gandi S., Joshi D. Nanostructured Metal Oxide Semiconductor-Based Gas Sensors: A Comprehensive Review. Sens. Actuators Phys. 2022;341:113578. doi: 10.1016/j.sna.2022.113578. - DOI
-
- Zhang C., Liu G., Geng X., Wu K., Debliquy M. Metal Oxide Semiconductors with Highly Concentrated Oxygen Vacancies for Gas Sensing Materials: A Review. Sens. Actuators Phys. 2020;309:112026. doi: 10.1016/j.sna.2020.112026. - DOI
-
- Wang M., Zhu Y., Luo Q., Ge C., Liu G., Qiao G., Kim E.J. Below-Room-Temperature Solution-Grown ZnO Porous Nanosheet Arrays with Ppb-Level NO2 Sensitivity under Intermittent UV Irradiation. Appl. Surf. Sci. 2021;566:150750. doi: 10.1016/j.apsusc.2021.150750. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

