Vasohibins in Health and Disease: From Angiogenesis to Tumorigenesis, Multiorgan Dysfunction, and Brain-Heart Remodeling
- PMID: 40497943
- PMCID: PMC12153568
- DOI: 10.3390/cells14110767
Vasohibins in Health and Disease: From Angiogenesis to Tumorigenesis, Multiorgan Dysfunction, and Brain-Heart Remodeling
Abstract
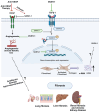
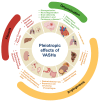
Vasohibins (VASHs), comprising VASH-1 and VASH-2, were initially identified as regulators of angiogenesis. Recent studies, however, have unveiled their novel role in fibrosis and microtubule detyrosination. The dysregulated expression of VASHs is associated with several pathological processes, such as angiogenesis dysfunction, microtubule detyrosination, and fibrosis, contributing to various diseases. These findings suggest the pleiotropic effects of VASHs in multiple organs and systems beyond angiogenesis. This review explores the molecular properties of VASHs and their emerging functions in tubulin carboxyl activity and microtubule detyrosination-key to brain and cardiac remodeling. We also discuss the potential therapeutic applications of their interference in diseases such as tumorigenesis, as well as renal-, reproductive-, and liver-related diseases.
Keywords: angiogenesis regulator; cardiac/brain remodeling; fibrosis TGF-β/SMAD pathway; microtubule detyrosination; vasohibins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
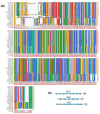
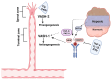

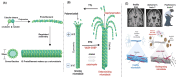
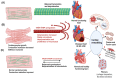
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

