Understanding Atherosclerotic Plaque Cellular Composition: Recent Advances Driven by Single Cell Omics
- PMID: 40497946
- PMCID: PMC12153806
- DOI: 10.3390/cells14110770
Understanding Atherosclerotic Plaque Cellular Composition: Recent Advances Driven by Single Cell Omics
Abstract
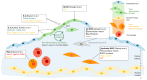
Atherosclerosis, a chronic inflammatory condition of the arterial cell wall, is the leading cause of cardiovascular disease (CVD) and death. It is characterised by the accumulation of lipid and immune cell-rich plaques in the arterial intima, which is driven by a dysregulated immune response to cholesterol-containing lipoproteins at the lesion site. Initially thought to be driven by passive lipid accumulation, atherosclerosis is now recognised as a complex process encompassing a multitude of inflammatory and remodelling mechanisms, driven by both immune cells (e.g., macrophages, T-cells, B-cells, antigen-presenting cells) and non-immune cells (smooth muscle cells, endothelial cells). With the development of single-cell transcriptomic and proteomic technologies, a previously inconceivable wealth of data has been generated in an attempt to better understand the pathophysiology of the disease and identify novel avenues for the development of targeted therapeutic interventions. This review provides an overview of the latest findings in the field obtained using single-cell technologies, with a focus on the major cell types present at the atherosclerotic plaque site, their suggested repartition in subsets, as well as their predicted function(s) within the complex processes that interplay to drive atherosclerotic disease. We conclude by highlighting the discrepancies and areas of consensus brought about by these studies and briefly discuss the likely future advances that will come from the continuous development and improvement of single-cell omics technologies.
Keywords: atherosclerosis; single-cell omics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Cochain C., Vafadarnejad E., Arampatzi P., Pelisek J., Winkels H., Ley K., Wolf D., Saliba A.-E., Zernecke A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 2018;122:1661–1674. doi: 10.1161/CIRCRESAHA.117.312509. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

