Dendritic Cell-Based Cancer Vaccines: The Impact of Modulating Innate Lymphoid Cells on Anti-Tumor Efficacy
- PMID: 40497988
- PMCID: PMC12155103
- DOI: 10.3390/cells14110812
Dendritic Cell-Based Cancer Vaccines: The Impact of Modulating Innate Lymphoid Cells on Anti-Tumor Efficacy
Abstract
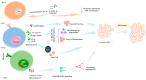
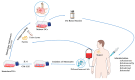
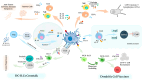
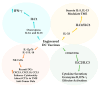
Dendritic cell (DC) vaccines stimulate the immune system to target cancer antigens, representing a promising option for immunotherapy. However, clinical trials have demonstrated limited effectiveness, emphasizing the need for enhanced immune responses. Improving the production of DC vaccines, assessing their impact on immune components, and observing responses could improve the results of DC-based therapies. Innate lymphoid cells (ILCs) represent a heterogeneous population of innate immune components that generate cytokines and modulate the immune system, potentially enhancing immunotherapies. Recent research highlights the different functions of ILCs in cancer, demonstrating their dual capabilities to promote tumors and exhibit anti-tumor actions. DCs and ILCs actively communicate under physiological and pathological conditions, and the activation of ILCs by DCs or DC vaccines has been shown to influence ILC cytokine production and function. Gaining insights into the interaction between DC-activated ILCs and tumors is essential for creating exciting new therapeutic strategies. These strategies aim to boost anti-tumor immunity while reducing the support that tumors receive. This review examines the effect of DC vaccination on host ILCs, illustrating the complex relationship between DC-based vaccines and ILCs. Furthermore, it explores some exciting strategies to enhance DC vaccines, aiming to boost anti-tumor immune responses by fostering better engagement with ILCs.
Keywords: dendritic cell (DC) vaccines; innate lymphoid cells (ILCs); tumor microenvironment (TME).
Conflict of interest statement
B.W.B. is the Chief Executive Officer of ImmunoCeutica Inc. (ICI), which is dedicated to the research and development of immunoceuticals. B.W.B. serves as a scientific advisor for the Canadian COVID Care Alliance (CCCA), Taking Back Our Freedoms (TBoF). Neither ICI, CCCA, nor TBoF was involved in any way with this manuscript and the research it describes. B.W.B. has received honoraria for speaking engagements and has provided paid expert testimony in court proceedings, utilizing his expertise in viral immunology. The other authors declare no potential conflicts of interest. The funder had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Dendritic Cell Vaccines Impact the Type 2 Innate Lymphoid Cell Population and Their Cytokine Generation in Mice.Vaccines (Basel). 2023 Oct 3;11(10):1559. doi: 10.3390/vaccines11101559. Vaccines (Basel). 2023. PMID: 37896962 Free PMC article.
-
Empowering dendritic cell cancer vaccination: the role of combinatorial strategies.Cytotherapy. 2018 Nov;20(11):1309-1323. doi: 10.1016/j.jcyt.2018.09.007. Epub 2018 Oct 22. Cytotherapy. 2018. PMID: 30360963 Review.
-
The Potential of Dendritic-Cell-Based Vaccines to Modulate Type 3 Innate Lymphoid Cell Populations.Int J Mol Sci. 2023 Jan 26;24(3):2403. doi: 10.3390/ijms24032403. Int J Mol Sci. 2023. PMID: 36768726 Free PMC article.
-
Innate lymphoid cell memory.Cell Mol Immunol. 2019 May;16(5):423-429. doi: 10.1038/s41423-019-0212-6. Epub 2019 Feb 22. Cell Mol Immunol. 2019. PMID: 30796350 Free PMC article. Review.
-
The subtle interplay between gamma delta T lymphocytes and dendritic cells: is there a role for a therapeutic cancer vaccine in the era of combinatorial strategies?Cancer Immunol Immunother. 2021 Jul;70(7):1797-1809. doi: 10.1007/s00262-020-02805-3. Epub 2021 Jan 1. Cancer Immunol Immunother. 2021. PMID: 33386466 Free PMC article. Review.
Cited by
-
Therapeutic Cancer Vaccines in Colorectal Cancer: Platforms, Mechanisms, and Combinations.Cancers (Basel). 2025 Aug 6;17(15):2582. doi: 10.3390/cancers17152582. Cancers (Basel). 2025. PMID: 40805277 Free PMC article.
-
Natural carrier systems in cancer vaccines and immunotherapy.Hum Vaccin Immunother. 2025 Dec;21(1):2535787. doi: 10.1080/21645515.2025.2535787. Epub 2025 Jul 24. Hum Vaccin Immunother. 2025. PMID: 40707012 Free PMC article. Review.
References
-
- Bubenik J. Dendritic cell-based cancer vaccines. Folia Biol. 1999;45:71–74. - PubMed
-
- Karimi K., Boudreau J.E., Fraser K., Liu H., Delanghe J., Gauldie J., Xing Z., Bramson J.L., Wan Y. Enhanced antitumor immunity elicited by dendritic cell vaccines is a result of their ability to engage both CTL and IFNγ-producing NK cells. Mol. Therapy. 2008;16:411–418. doi: 10.1038/sj.mt.6300347. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

