Efficient Generation of Induced Pluripotent Stem Cell-Derived Definitive Endoderm Cells with Growth Factors and Small Molecules
- PMID: 40497991
- PMCID: PMC12153844
- DOI: 10.3390/cells14110815
Efficient Generation of Induced Pluripotent Stem Cell-Derived Definitive Endoderm Cells with Growth Factors and Small Molecules
Abstract
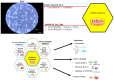
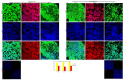
Definitive endoderm (DE) differentiation leads to the development of the major internal organs including the liver, intestines, pancreas, gall bladder, prostate, bladder, thyroid, and lungs. The two primary methods utilized for in vitro differentiation of induced pluripotent stem cells (iPSCs) into DE cells are the growth factor (GF) and the small molecule (SM) approaches. The GSK-3 inhibitor (CHIR99021) is a key factor for the SM approach. Activin A and Wnt3a are utilized in the GF approach. In this study, both the GF and SM protocols were compared to each other. The results show that both the GF and SM protocol produce DE with a similar morphological phenotype, gene and protein expression, and a similar level of homogeneity and functionality. However, on both the gene expression and proteomic level, there is a divergence between the two protocols during hepatic specification. Proteomic analysis shows that hepatoblasts from the GF protocol have significantly differentially expressed proteins (DEPs) involved in liver metabolic pathways compared to the SM protocol. Well-validated DE differentiation protocols are needed to fully unlock the clinical potential of iPSCs. In the first step of generating DE-derived tissue, either protocol can be utilized. However, for hepatic specification, the GF protocol is more effective.
Keywords: definitive endoderm; growth factor; induced pluripotent stem cell cells; small molecule.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Molè M.A., Coorens T.H.H., Shahbazi M.N., Weberling A., Weatherbee B.A.T., Gantner C.W., Sancho-Serra C., Richardson L., Drinkwater A., Syed N., et al. A single cell characterization of human embryogenesis identifies pluripotency transitions and putative anterior hypoblast center. Nat. Commun. 2021;12:3679. doi: 10.1038/s41467-021-23758-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

