Isolation and Characterization of Articular Cartilage-Derived Cells Obtained by Arthroscopic Cartilage Biopsy from Non-Osteoarthritic Patients
- PMID: 40498006
- PMCID: PMC12155265
- DOI: 10.3390/cells14110830
Isolation and Characterization of Articular Cartilage-Derived Cells Obtained by Arthroscopic Cartilage Biopsy from Non-Osteoarthritic Patients
Abstract
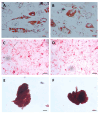
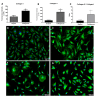
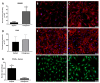
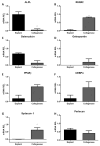
Cartilage-derived migratory cells show great potential for autologous use in cartilage repair surgery. However, their collection through arthroscopic biopsy has not been previously reported in individuals without osteoarthritis. This study aimed to characterize migratory cartilage cells isolated from arthroscopic biopsies of volunteers without osteoarthritis and compare them with cells obtained by enzymatic digestion. Cell cultures were successfully established using both methods-enzymatic digestion and cell migration-from cartilage explants, with no significant differences observed in stem cell markers or plasticity between the cell lines. Cells derived from both procedures exhibited characteristics of mesenchymal stem cell, including fibroblast-like morphology, expression of CD29, CD90, and CD105 markers, absence of hematopoietic and endothelial cell markers, and the ability to differentiate into adipocytes, chondrocytes, and osteoblasts under appropriate conditions. Cells obtained by migration showed lower expression of collagen I and II, along with reduce collagen II/collagen I ratio, both positively associated with chondral matrix production, as well as lower RUNX2 expression. However, no differences were found in the levels of SOX9, essential for chondrogenic differentiation, or in the expression of perlecan gene. Syndecan-1 expression was lower in cells obtained by migration. In conclusion, this study demonstrates that cartilage-derived migratory cells can be successfully obtained from arthroscopic biopsies of individuals without osteoarthritis, presenting comparable dedifferentiation and plasticity profiles. Furthermore, these cells express essential chondrogenic markers and proteins. Although further in vivo studies are needed to determine their effective regenerative potential, cartilage-derived migratory cells represent a promising avenue for cartilage repair strategies.
Keywords: cartilage explants; cartilage repair; cartilage-derived cells; enzymatic cartilage digestion; mesenchymal stem cells.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Hinckel B.B., Thomas D., Vellios E.E., Hancock K.J., Calcei J.G., Sherman S.L., Eliasberg C.D., Fernandes T.L., Farr J., Lattermann C., et al. Algorithm for Treatment of Focal Cartilage Defects of the Knee: Classic and New Procedures. Cartilage. 2021;13:473S–495S. doi: 10.1177/1947603521993219. - DOI - PMC - PubMed
-
- Matta C., Boocock D.J., Fellows C.R., Miosge N., Dixon J.E., Liddell S., Smith J., Mobasheri A. Molecular phenotyping of the surfaceome of migratory chondroprogenitors and mesenchymal stem cells using biotinylation, glycocapture and quantitative LC-MS/MS proteomic analysis. Sci. Rep. 2019;9:9018. doi: 10.1038/s41598-019-44957-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

