Investigating the Immunogenic Potential of Variations in Host Cell Protein Levels in Clinical-Grade AAV8 Products
- PMID: 40498045
- PMCID: PMC12166503
- DOI: 10.1167/iovs.66.6.38
Investigating the Immunogenic Potential of Variations in Host Cell Protein Levels in Clinical-Grade AAV8 Products
Abstract
Purpose: Adeno-associated virus (AAV) formulations for gene therapy contain manufacturing-associated impurities such as residual host cell protein (HCP). The aim of this study was to investigate whether high levels of HCP in AAV formulations are associated with increased inflammation and reduced ocular tolerability.
Methods: Three lots of clinical-grade AAV8 vector were analyzed for the presence of manufacturing-associated impurities. The HCP component of these impurities was characterized using mass spectrometry. Lots were then compared regarding their capacity to induce a cytokine response in primary human plasmacytoid dendritic cells (pDCs) and THP-1 cells. Furthermore, the results of an ocular safety study in healthy nonhuman primates were analyzed post hoc to investigate the influence of HCP levels on clinical signs of inflammation and chorioretinal atrophy (CRA) development.
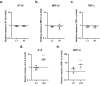
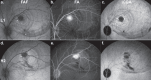
Results: Vector lots displayed up to a ∼40-fold variation in HCP levels. Human galactin-3-binding protein was the only major HCP contaminant. Stimulation of human pDCs and THP-1 cells with a high HCP lot did not result in an increased cytokine response. High HCP also did not exacerbate clinical signs of inflammation. However, on retinal imaging, CRA lesions were significantly larger in high HCP-treated eyes (P = 0.001-0.048).
Conclusions: HCP impurities were of low complexity, but pronounced variations in their abundance were observed between lots. High HCP levels were not overtly immunogenic in vivo and in vitro. However, despite statistical limitations, they seemed to be associated with increased CRA. Thus, a negative effect of high HCP levels on retinal tolerability could not be ruled out.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Extra-viral DNA in adeno-associated viral vector preparations induces TLR9-dependent innate immune responses in human plasmacytoid dendritic cells.Sci Rep. 2023 Feb 2;13(1):1890. doi: 10.1038/s41598-023-28830-7. Sci Rep. 2023. PMID: 36732401 Free PMC article.
-
AAV8 Can Induce Innate and Adaptive Immune Response in the Primate Eye.Mol Ther. 2017 Dec 6;25(12):2648-2660. doi: 10.1016/j.ymthe.2017.08.018. Epub 2017 Aug 31. Mol Ther. 2017. PMID: 28970046 Free PMC article.
-
Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells.J Virol. 2006 Dec;80(24):11899-910. doi: 10.1128/JVI.00890-06. Epub 2006 Sep 27. J Virol. 2006. PMID: 17005662 Free PMC article.
-
Adeno-associated virus (AAV) vectors in gene therapy: immune challenges and strategies to circumvent them.Rev Med Virol. 2013 Nov;23(6):399-413. doi: 10.1002/rmv.1762. Epub 2013 Sep 10. Rev Med Virol. 2013. PMID: 24023004 Review.
-
Immune responses to AAV vectors: overcoming barriers to successful gene therapy.Blood. 2013 Jul 4;122(1):23-36. doi: 10.1182/blood-2013-01-306647. Epub 2013 Apr 17. Blood. 2013. PMID: 23596044 Free PMC article. Review.
References
-
- Reichel FF, Seitz I, Wozar F, et al.. Development of retinal atrophy after subretinal gene therapy with voretigene neparvovec. Br J Ophthalmol. 2023; 107(9): 1331–1335. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

