Impacts of the Mindfulness Meditation Mobile App Calm on Undergraduate Students' Sleep and Emotional State: Pilot Randomized Controlled Trial
- PMID: 40498669
- PMCID: PMC12175875
- DOI: 10.2196/66131
Impacts of the Mindfulness Meditation Mobile App Calm on Undergraduate Students' Sleep and Emotional State: Pilot Randomized Controlled Trial
Abstract
Background: Undergraduate students frequently experience negative emotional states and sleep quality, which is believed to have worsened following the COVID-19 pandemic.
Objective: This study piloted the use of a popular mobile mindfulness app (Calm) as a potential intervention to improve state depression, anxiety, stress, and sleep quality in undergraduate students attending a Canadian university, following the COVID-19 pandemic.
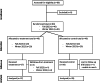
Methods: Undergraduate students were randomly assigned to a control or treatment group and completed a series of 3 questionnaires to evaluate baseline state emotional health (Depression Anxiety Stress Scale 42-Item Version [DASS-42], Perceived Stress Scale 10-Item Version [PSS-10], and Pittsburgh Sleep Quality Index). Treatment group participants were instructed to engage with the fully-automated Calm app's sleep section for 30 days: 20 minutes daily, 5 days a week, along with an additional 30 minutes of interaction with other app sections each week, resulting in a goal of 130 minutes per week. The control participants were instructed to continue with everyday life and refrain from the use of mindfulness-based apps for 30 days. Following the 30-day treatment period, all participants repeated the 3 questionnaires. The impact of the treatment on all outcomes was examined using linear mixed model analyses. Independent samples t tests were used to determine if psychosocial health or sleep scores differed between baseline and follow-up and if differences in such scores were present between the groups.
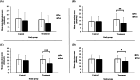
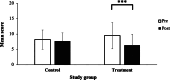
Results: A total of 80 students met the inclusion criteria and were randomly assigned to the control (n=40) or treatment (n=40) group. One control participant was lost to follow-up and 3 treatment participants discontinued engaging with the Calm app. Both control (n=39) and treatment (n=37) groups began with similar demographic, emotional state, and sleep characteristics. Treatment participants engaged with the Calm app's sleep section for an average of 234 minutes per week; however, 54% (20/37) met the minimum prescribed interaction time across all 4 weeks. Following the 30-day treatment period, compared to the control group, the treatment group's state anxiety (mean 14, SD 7.4 vs mean 12, SD 7.8; P=.002), state stress (DASS-42: mean 20, SD 8.8 vs mean 15, SD 8.5; P<.001; PSS-10: mean 22, SD 5.9 vs mean 19, SD 5.9; P=.02), and sleep quality (mean 7.7, SD 2.7 vs mean 6.4, SD 3.5; P<.001) improved. Posttreatment, state stress and perceived stress severity was lower in the treatment versus control group (DASS-42: P=.02; PSS-10: P=.03, respectively).
Conclusions: These pilot findings indicate that a mindfulness app may be an effective tool for reducing state anxiety and stress, as well as enhancing sleep quality among undergraduate university students. A larger, randomized controlled trial should confirm these findings.
Keywords: anxiety; depression; emotional state; mHealth; mindfulness; mobile apps; mobile health; mobile phone; pilot study; sleep; stress; students; university.
© Tovan Lew, Natnaiel M Dubale, Erik Doose, Alex Adenuga, Holly E Bates, Sarah L West. Originally published in JMIR Formative Research (https://formative.jmir.org).
Conflict of interest statement
Figures

Similar articles
-
Mindful moms: acceptability and impact of co-designed and digitally delivered video meditations for pregnant and parenting women with opioid use disorder.Ann Med. 2025 Dec;57(1):2486585. doi: 10.1080/07853890.2025.2486585. Epub 2025 Apr 18. Ann Med. 2025. PMID: 40248919 Free PMC article. Clinical Trial.
-
An examination of the effectiveness of mindfulness-integrated cognitive behavior therapy on depression, anxiety, stress and sleep quality in Iranian women with breast cancer: a randomized controlled trial.Sci Rep. 2025 Apr 1;15(1):11041. doi: 10.1038/s41598-025-85745-1. Sci Rep. 2025. PMID: 40169689 Free PMC article. Clinical Trial.
-
Effectiveness of an online mindfulness based stress reduction intervention on psychological distress among patients with COVID19 after hospital discharge.Sci Rep. 2025 Jul 13;15(1):25325. doi: 10.1038/s41598-025-11289-z. Sci Rep. 2025. PMID: 40653580 Free PMC article. Clinical Trial.
-
Pre-deployment programmes for building resilience in military and frontline emergency service personnel.Cochrane Database Syst Rev. 2021 Dec 6;12(12):CD013242. doi: 10.1002/14651858.CD013242.pub2. Cochrane Database Syst Rev. 2021. PMID: 34870330 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
References
-
- Blanco C, Okuda M, Wright C, et al. Mental health of college students and their non-college-attending peers: results from the National Epidemiologic Study on Alcohol and Related Conditions. Arch Gen Psychiatry. 2008 Dec;65(12):1429–1437. doi: 10.1001/archpsyc.65.12.1429. doi. Medline. - DOI - PMC - PubMed
-
- Stallman HM. Psychological distress in university students: a comparison with general population data. Aust Psychol. 2010 Dec 1;45(4):249–257. doi: 10.1080/00050067.2010.482109. doi. - DOI
-
- Hamza CA, Ewing L, Heath NL, Goldstein AL. When social isolation is nothing new: a longitudinal study on psychological distress during COVID-19 among university students with and without preexisting mental health concerns. Can Psychol / Psychol Can. 2021;62(1):20–30. doi: 10.1037/cap0000255. doi. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

