Detectable SARS-CoV-2 specific immune responses in recovered unvaccinated individuals 250 days post wild type infection
- PMID: 40498720
- PMCID: PMC12157120
- DOI: 10.1371/journal.pone.0325923
Detectable SARS-CoV-2 specific immune responses in recovered unvaccinated individuals 250 days post wild type infection
Abstract
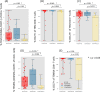
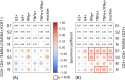
Memory T cells play an important role in mediating long-lasting adaptive immune responses to viral infections, such as SARS-CoV-2. In the context of the latter, much of our current knowledge stems from studies in vaccinated individuals or repeatedly infected individuals. However, limited knowledge is available on these responses in fully naive individuals in German communities. We performed immunophenotyping of a previously naive SARS-CoV-2 cohort in convalescent individuals after asymptomatic to moderate COVID-19. The samples were collected median 250 days post infection during the first wave of the COVID pandemic in Germany (March - May 2020). In this cohort of 174 individuals, we phenotyped different leukocyte cell populations in peripheral blood (B, T and Natural Killer cells). We then assessed the serostatus against the SARS-CoV-2 antigens Nucleocapsid (N) and Spike subunit (S1) with its receptor binding domain (RBD), as these are important correlates of protection, by testing for presence of immunoglobulin G (IgG) antibodies. We also measured IgG antibody responses against the N antigen of the common cold coronaviruses HCoV-OC43, HCoV-HKU1, HCoV-NL63 and HCoV-229E, to determine possible cross-reactivity. In a subset of the cohort (n = 76), we performed intracellular staining assays (ICS) after stimulation with SARS-CoV-2 and HCoV antigens. Key findings are significant differences in frequency of CD4+ memory T cell populations, notably CD4+ TEM and CD4+ TEMRA cells, between the group of SARS-CoV-2 positive individuals and the control group. These differences correlated with cytokine production (TNFα, IFNγ) after stimulation with SARS-CoV-2 peptides, indicating a specific T cell immune response. In conclusion, a clear memory T cell and humoral response can be detected up to 250 days post mild to moderate COVID-19 disease. Our results underline findings reported by others indicating a lasting cellular immune response even in a population which previously had not been exposed to SARS-CoV-2.
Copyright: © 2025 Weigl et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
“We have read the journal’s policy and the authors of this manuscript have the following competing interests, which are unrelated to the topic of the present manuscript but broadly relevant to medical research and thus be perceived as a lack of transparency if not disclosed: S.K. has received honoraria from Cymab, Plectonic, TCR2 Inc., Miltenyi, Galapagos, Novartis, BMS and GSK. S. K. is an inventor of several patents in the field of immuno-oncology. S. K. received license fees from TCR2 Inc and Carina Biotech. S.K. received research support from TCR2 Inc., Tabby Therapeutics, Catalym GmBH, Plectonic GmBH and Arcus Bioscience for work unrelated to the manuscript. The remaining authors declare that they have no conflicts of interest that might be relevant to the contents of this manuscript. The mentioned companies Cymab, Plectonic, TCR2 Inc., Miltenyi, Galapagos, Novartis, BMS and GSK etc. did not have any role in conceptualization or content of the study. As these companies are broadly relevant in the medical field their interactions with S.K. were mentioned for transparency reasons. These interactions do not alter our adherence to PLOS ONE policies on sharing data and materials. The patents S.K. holds in the field of immuno-oncology also do not have a connection to the conceptualization or content of the study, it does not alter our adherence to PLOS ONE policies on sharing data and materials.”
Figures


References
-
- WHO - World Health Organisation. Coronavirus disease (COVID-19) pandemic 2024. Available from: https://www.who.int/europe/emergencies/situations/covid-19
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

