Remimazolam-etomidate versus remimazolam-propofol for gastrointestinal endoscopy: A randomized controlled trial
- PMID: 40498785
- PMCID: PMC12157239
- DOI: 10.1371/journal.pone.0326043
Remimazolam-etomidate versus remimazolam-propofol for gastrointestinal endoscopy: A randomized controlled trial
Abstract
Objective: The optimal sedation strategy for gastrointestinal endoscopy remains debated. This study compared the efficacy and safety of remimazolam combined with etomidate versus propofol for procedural sedation during gastrointestinal endoscopy.
Methods: This single-center, randomized controlled clinical trial was performed from March 2024 to April 2024. A total of 262 patients scheduled to undergo gastrointestinal endoscopy were randomly assigned to receive remimazolam-etomidate (RE) or remimazolam-propofol (RP). The primary outcome was the incidence of respiratory depression. Secondary outcomes included the results of sedation and recovery. The safety results mainly include the incidence of hypotension, bradycardia, tachycardia, painful injection and muscular tremor. Statistical analyses included t-tests, Mann-Whitney U tests, and χ² tests for group comparisons, with subgroup analyses and multivariable logistic regression to assess the robustness of primary outcome.
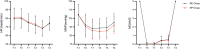
Results: Respiratory depression occurred in 20.0% (25/125) of RE patients versus 32.3% (40/124) of RP patients (OR=0.52; 95% CI = 0.29-0.93; p = 0.028). There was a statistically significant difference in the distribution of the number of airway interventions between the two groups (p = 0.043), with 18 patients (14.5%) in the RP group requiring three airway interventions and only seven patients (5.6%) in the RE group. Hypoxemia occurred in three patients (2.4%) in the RE group and in five patients (4.0%) in the RP group. Hypotension was observed in 23.2% of patients sedated with RE versus 36.3% of patients sedated with RP (p = 0.024).
Conclusion: Remimazolam-etomidate demonstrated a superior safety profile, with reduced respiratory depression and hemodynamic instability compared to remimazolam-propofol, suggesting its potential as a safer alternative for gastrointestinal endoscopy sedation.
Registration information: This trial was prospectively registered at the Chinese Clinical Trial Registry (ChiCTR2400085904) prior to patient enrollment.
Copyright: © 2025 Ma et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Etomidate Combined with Propofol versus Remimazolam for Sedation in Elderly Patients During Gastrointestinal Endoscopy: A Randomized Prospective Clinical Trial.Drug Des Devel Ther. 2024 Jul 2;18:2681-2692. doi: 10.2147/DDDT.S454314. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38974124 Free PMC article. Clinical Trial.
-
Remimazolam versus propofol for endoscopy sedation in elderly patients: a systematic review, meta-analysis and trial sequential analysis.Minerva Anestesiol. 2024 Sep;90(9):775-784. doi: 10.23736/S0375-9393.24.18027-3. Epub 2024 May 22. Minerva Anestesiol. 2024. PMID: 38775443
-
Effectiveness and safety of remimazolam tosilate versus propofol for sedation in patients undergoing gastrointestinal endoscopy: a randomized controlled trial.Int J Clin Pharm. 2024 Dec;46(6):1371-1380. doi: 10.1007/s11096-024-01774-2. Epub 2024 Jul 31. Int J Clin Pharm. 2024. PMID: 39083220 Clinical Trial.
-
Remimazolam tosilate in upper gastrointestinal endoscopy: A multicenter, randomized, non-inferiority, phase III trial.J Gastroenterol Hepatol. 2021 Feb;36(2):474-481. doi: 10.1111/jgh.15188. Epub 2020 Jul 24. J Gastroenterol Hepatol. 2021. PMID: 32677707 Clinical Trial.
-
Remimazolam versus propofol for sedation in gastrointestinal endoscopic procedures: a systematic review and meta-analysis.Br J Anaesth. 2024 Jun;132(6):1219-1229. doi: 10.1016/j.bja.2024.02.005. Epub 2024 Mar 4. Br J Anaesth. 2024. PMID: 38443286
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

