A lipid atlas of the human kidney
- PMID: 40498844
- PMCID: PMC12154186
- DOI: 10.1126/sciadv.adu3730
A lipid atlas of the human kidney
Abstract
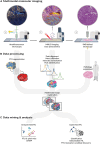
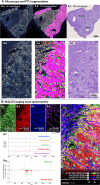
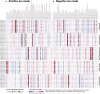
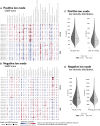
Tissue atlases provide foundational knowledge on the cellular organization and molecular distributions across molecular classes and spatial scales. Here, we construct a comprehensive spatiomolecular lipid atlas of the human kidney from 29 donor tissues using integrated multimodal molecular imaging. Our approach leverages high-spatial-resolution matrix-assisted laser desorption/ionization imaging mass spectrometry for untargeted lipid mapping, stained microscopy for histopathological assessment, and tissue segmentation using autofluorescence microscopy. With a combination of unsupervised, supervised, and interpretable machine learning, the atlas provides multivariate lipid profiles of specific multicellular functional tissue units (FTUs) of the nephron, including the glomerulus, proximal tubules, thick ascending limb, distal tubules, and collecting ducts. In total, the atlas consists of tens of thousands of FTUs and millions of mass spectrometry measurements. Detailed patient, clinical, and histopathologic information allowed molecular data to be mined on the basis of these features. As examples, we highlight the discovery of how lipid profiles are altered with sex and differences in body mass index.
Figures



References
-
- Conde C. D., Xu C., Jarvis L. B., Rainbow D. B., Wells S. B., Gomes T., Howlett S. K., Suchanek O., Polanski K., King H. W., Mamanova L., Huang N., Szabo P. A., Richardson L., Bolt L., Fasouli E. S., Mahbubani K. T., Prete M., Tuck L., Richoz N., Tuong Z. K., Campos L., Mousa H. S., Needham E. J., Pritchard S., Li T., Elmentaite R., Park J., Rahmani E., Chen D., Menon D. K., Bayraktar O. A., James L. K., Meyer K. B., Yosef N., Clatworthy M. R., Sims P. A., Farber D. L., Saeb-Parsy K., Jones J. L., Teichmann S. A., Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science 376, eabl5197 (2022). - PMC - PubMed
-
- The Tabula Sapiens Consortium, Jones R. C., Karkanias J., Krasnow M. A., Pisco A. O., Quake S. R., Salzman J., Yosef N., Bulthaup B., Brown P., Harper W., Hemenez M., Ponnusamy R., Salehi A., Sanagavarapu B. A., Spallino E., Aaron K. A., Concepcion W., Gardner J. M., Kelly B., Neidlinger N., Wang Z., Crasta S., Kolluru S., Morri M., Tan S. Y., Travaglini K. J., Xu C., Alcántara-Hernández M., Almanzar N., Antony J., Beyersdorf B., Burhan D., Calcuttawala K., Carter M. M., Chan C. K. F., Chang C. A., Chang S., Colville A., Culver R. N., Cvijović I., D’Amato G., Ezran C., Galdos F. X., Gillich A., Goodyer W. R., Hang Y., Hayashi A., Houshdaran S., Huang X., Irwin J. C., Jang S., Juanico J. V., Kershner A. M., Kim S., Kiss B., Kong W., Kumar M. E., Kuo A. H., Leylek R., Li B., Loeb G. B., Lu W.-J., Mantri S., Markovic M., McAlpine P. L., de Morree A., Mrouj K., Mukherjee S., Muser T., Neuhöfer P., Nguyen T. D., Perez K., Phansalkar R., Puluca N., Qi Z., Rao P., Raquer-McKay H., Schaum N., Scott B., Seddighzadeh B., Segal J., Sen S., Sikandar S., Spencer S. P., Steffes L. C., Subramaniam V. R., Swarup A., Swift M., Van Treuren W., Trimm E., Veizades S., Vijayakumar S., Vo K. C., Vorperian S. K., Wang W., Weinstein H. N. W., Winkler J., Wu T. T. H., Xie J., Yung A. R., Zhang Y., Detweiler A. M., Mekonen H., Neff N. F., Sit R. V., Tan M., Yan J., Bean G. R., Charu V., Forgó E., Martin B. A., Ozawa M. G., Silva O., Toland A., Vemuri V. N. P., Afik S., Awayan K., Botvinnik O. B., Byrne A., Chen M., Dehghannasiri R., Gayoso A., Granados A. A., Li Q., Mahmoudabadi G., McGeever A., Olivieri J. E., Park M., Ravikumar N., Stanley G., Tan W., Tarashansky A. J., Vanheusden R., Wang P., S. Wang, Xing G., Dethlefsen L., Ho P.-Y., Liu S., Maltzman J. S., Metzger R. J., Sasagawa K., Sinha R., Song H., Wang B., Artandi S. E., Beachy P. A., Clarke M. F., Giudice L. C., Huang F. W., Huang K. C., Idoyaga J., Kim S. K., Kuo C. S., Nguyen P., Rando T. A., Red-Horse K., Reiter J., Relman D. A., Sonnenburg J. L., Wu A., Wu S. M., Wyss-Coray T., The Tabula Sapiens: A multiple-organ, single-cell transcriptomic atlas of humans. Science 376, eabl4896 (2022). - PMC - PubMed
-
- Eraslan G., Drokhlyansky E., Anand S., Fiskin E., Subramanian A., Slyper M., Wang J., Van Wittenberghe N., Rouhana J. M., Waldman J., Ashenberg O., Lek M., Dionne D., Win T. S., Cuoco M. S., Kuksenko O., Tsankov A. M., Branton P. A., Marshall J. L., Greka A., Getz G., Segrè A. V., Aguet F., Rozenblatt-Rosen O., Ardlie K. G., Regev A., Single-nucleus cross-tissue molecular reference maps toward understanding disease gene function. Science 376, eabl4290 (2022). - PMC - PubMed
-
- Hansen J., Sealfon R., Menon R., Eadon M. T., Lake B. B., Steck B., Anjani K., Parikh S., Sigdel T. K., Zhang G., Velickovic D., Barwinska D., Alexandrov T., Dobi D., Rashmi P., Otto E. A., Rivera M., Rose M. P., Anderton C. R., Shapiro J. P., Pamreddy A., Winfree S., Xiong Y., He Y., de Boer I. H., Hodgin J. B., Barisoni L., Naik A. S., Sharma K., Sarwal M. M., Zhang K., Himmelfarb J., Rovin B., El-Achkar T. M., Laszik Z., He J. C., Dagher P. C., Valerius M. T., Jain S., Satlin L. M., Troyanskaya O. G., Kretzler M., Iyengar R., Azeloglu E. U., Kidney Precision Medicine Project , A reference tissue atlas for the human kidney. Sci. Adv. 8, eabn4965 (2022). - PMC - PubMed
-
- Lake B. B., Menon R., Winfree S., Hu Q., Ferreira R. M., Kalhor K., Barwinska D., Otto E. A., Ferkowicz M., Diep D., Plongthongkum N., Knoten A., Urata S., Mariani L. H., Naik A. S., Eddy S., Zhang B., Wu Y., Salamon D., Williams J. C., Wang X., Balderrama K. S., Hoover P. J., Murray E., Marshall J. L., Noel T., Vijayan A., Hartman A., Chen F., Waikar S. S., Rosas S. E., Wilson F. P., Palevsky P. M., Kiryluk K., Sedor J. R., Toto R. D., Parikh C. R., Kim E. H., Satija R., Greka A., Macosko E. Z., Kharchenko P. V., Gaut J. P., Hodgin J. B., KPMP Consortium, Eadon M. T., Dagher P. C., El-Achkar T. M., Zhang K., Kretzler M., Jain S., An atlas of healthy and injured cell states and niches in the human kidney. Nature 619, 585–594 (2023). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

