Enhancing Thrombolysis Safety in Post-Acute Ischemic Stroke with Tissue Plasminogen Activator-Associated Microparticles
- PMID: 40498863
- PMCID: PMC12224294
- DOI: 10.1021/acsnano.5c01499
Enhancing Thrombolysis Safety in Post-Acute Ischemic Stroke with Tissue Plasminogen Activator-Associated Microparticles
Abstract
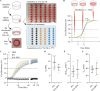
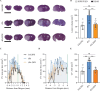
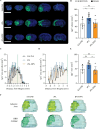
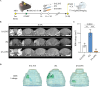
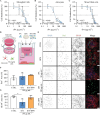
Recombinant tissue-type plasminogen activator (tPA) is the only approved thrombolytic drug for acute ischemic stroke, a condition associated with severe disabilities and high mortality. However, when the blood-brain barrier (BBB) is damaged, tPA can exacerbate cerebral injury and increase the risk of hemorrhagic transformation, limiting its use to a small subset of patients. To address this challenge and minimize extravascular accumulation, we combined tPA with micrometer-sized particles (DPN). We then tested their safety and neuroprotective effects. After a 1 h transient occlusion of the middle cerebral artery, free tPA, tPA-DPN, or saline was administered to assess mice survival, neurological behavior, and infarcted area extent. Free-tPA exacerbated brain damage, resulting in a modest 10% survival rate at 24 h post intervention. Conversely, tPA-DPN displayed a far better prognosis, with a 75% survival rate comparable to that of saline. No statistical differences were documented between tPA-DPN and saline for the Activity Score and the Neurological Severity Score. tPA-DPN did not increase lesion volume or BBB permeability, unlike free-tPA, which led to an over 2-fold enlarged lesion volume and 50% higher BBB permeability. The safety profile of tPA-DPN is attributed to the robust conjugation of tPA onto DPN and the lack of DPN extravasation, resulting in negligible cerebrovascular damage of free tPA and glial and neuron impairment. The vascular confinement of tPA linked to microscopic particles reduces drug side effects and represents a valuable strategy for safe and effective tPA delivery, even in the postacute stroke phase.
Keywords: blood–brain barrier; glial cells; nanomedicine; neuroprotection; thrombolytics.
Figures




References
-
- Sacco R. L., Kasner S. E., Broderick J. P., Caplan L. R., Connors J., Culebras A., Elkind M. S., George M. G., Hamdan A. D., Higashida R. T.. et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/str.0b013e318296aeca. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

