Gut Microbiota Modulation through Akkermansia spp. Supplementation Increases CAR T-cell Potency
- PMID: 40498998
- PMCID: PMC12409280
- DOI: 10.1158/2159-8290.CD-24-1230
Gut Microbiota Modulation through Akkermansia spp. Supplementation Increases CAR T-cell Potency
Abstract
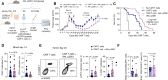
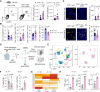
This study investigates the clinical relevance of the gut microbiome at taxonomic and metabolic levels in anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, both in patients and in a preclinical syngeneic tumor model. Patients with B-cell lymphoma treated with CD19 CAR T cells exhibited profound intestinal dysbiosis, exacerbated after CAR T-cell infusion. This dysbiosis was characterized by low bacterial richness, low soluble MAdCAM-1, and loss of Akkermansia species, associated with resistance to therapy. Mechanistically, oral Akkermansia massiliensis supplementation increased CAR T-cell infiltration into the bone marrow, inverted the CD4/CD8 CAR T-cell ratio, favored Tc1 CD8+ T-cell polarization, and promoted the release of tryptophan-derived indole metabolites, leading to better tumor control. The clinical benefit of Akkermansia spp. supplementation was abolished when CAR T cells were genetically deficient in the indole receptor, aryl hydrocarbon receptor (AhR). AhR-agonistic indoles alone failed to replicate the bacterium's anticancer effects. These findings suggest that Akkermansia supplementation could improve CAR T-cell potency in patients with intestinal Akkermansia deficiency.
Significance: B-cell lymphoma patients treated with CAR T cells harbor major gut microbiota perturbations and related metabolism that restrain CAR T-cell therapy. Reprogramming the gut microbiota ecosystem by oral A. massiliensis supplementation induces CAR T-cell niching and Tc1 differentiation in the bone marrow, promoting tumor control in an AhR-dependent manner.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A. Gottschlich reports grants from Tabby Therapeutics and Nanogami and nonfinancial support from Kite/Gilead outside the submitted work. L. Derosa reports being a consultant and scientific advisory board member for Bristol Myers Squibb and EverImmune. G. Kroemer reports grants from Daiichi Sankyo, Kaleido, Lytix Pharma, PharmaMar, Sanofi, Sutro, Tollys, and Vascage; grants and personal fees from Osasuna Therapeutics and Samsara Therapeutics; personal fees from Centenara Labs and Hevolution; and nonfinancial support from Institut Servier outside the submitted work and that he is on the Board of Directors of the Bristol Myers Squibb Foundation France and is a scientific co-founder of EverImmune, Osasuna Therapeutics, Samsara Therapeutics, and Therafast Bio. S. Kobold reports grants and personal fees from Plectonic and Tcr2; grants from Tabby, Arcus, and Catalym; and personal fees from Cymab, Bristol Myers Squibb, Miltenyi Biomedicines, Novartis, and GSK outside the submitted work. C. Castilla Llorente reports personal fees from Gilead/Kite outside the submitted work. H. Sokol reports other support from Exeliom Biosciences outside the submitted work. L. Zitvogel reports personal fees from EverImmune and grants from PiLeJe during the conduct of the study as well as grants from Daiichi Sankyo, personal fees from German Cancer Aid, and nonfinancial support from INCA outside the submitted work and has a patent for EP 23306837.8 pending to EverImmune. C. Bigenwald reports personal fees from Kite/Gilead and Bristol Myers Squibb during the conduct of the study and grants from Janssen outside the submitted work and has a patent for B23-5209QT pending. No disclosures were reported by the other authors.
Figures




References
-
- Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022;399:2294–308. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, Lunning M, Wang M, Arnason J, et al. Two-year follow-up of lisocabtagene maraleucel in relapsed or refractory large B-cell lymphoma in TRANSCEND NHL 001. Blood 2024;143:404–16. - PubMed
-
- Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med 2021;386:640–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

