Controlling the Helicity and Handedness of Polyaromatics with Isobenzofuranophane
- PMID: 40499034
- PMCID: PMC12338401
- DOI: 10.1002/anie.202510423
Controlling the Helicity and Handedness of Polyaromatics with Isobenzofuranophane
Abstract
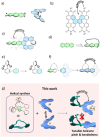
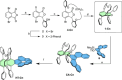
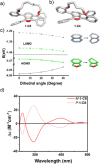
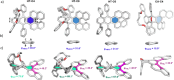
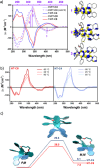
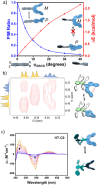
The extent of helicity in nonplanar polyaromatic materials affects their electronic, optical and chiroptical properties. However, controlling helicity in different systems typically requires different multistep synthetic approaches for each target compound, often with significant complexity, thereby limiting its applicability. Here, we introduce a helical synthon, isobenzofuranophane, with either a short or long tether, which enables late-stage helicity induction and control in aromatic frameworks. We demonstrate the applicability of this synthon by reacting it with a [5]helicene aryne precursor, where both the handedness and helicene pitch are remotely-controlled by the tether length, directly affecting the (chiro)optical properties of the helicene. The X-ray structures support these findings with up to 35° torsion for a single benzene ring, demonstrating the potential of isobenzofuranophane to induce significant curvature to polyaromatic molecules.
Keywords: Chirality; Circular dichroism; Circularly; Cyclophanes; Helicenes; Twistacenes.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Günther K., Grabicki N., Battistella B., Grubert L., Dumele O., J. Am. Chem. Soc. 2022, 144, 8707–8716. - PubMed
-
- Crassous J., Fuchter M. J., Freedman D. E., Kotov N. A., Moon J., Beard M. C., Feldmann S., Nat. Rev. Mater. 2023, 8, 365–371.
-
- Furlan F., Moreno‐Naranjo J. M., Gasparini N., Feldmann S., Wade J., Fuchter M. J., Nat. Photon. 2024, 18, 658–668.
-
- Delage‐Laurin L., Reger D., Suleymanov A., Nelson Z., Minion L., Kooi S., Brandt J., Siligardi G., Cameron R., Wade J., Swager T., Fuchter M., 2025, 10.26434/chemrxiv-2025-0ft6r. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

