Recommendations for Successful Development and Implementation of Digital Health Technology Tools
- PMID: 40499040
- PMCID: PMC12176242
- DOI: 10.2196/56747
Recommendations for Successful Development and Implementation of Digital Health Technology Tools
Abstract
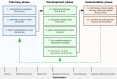
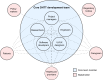
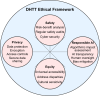
Digital health technology tools (DHTTs) have the potential to transform health care delivery by enabling new forms of participatory and personalized care that fit into patients' daily lives. However, realizing this potential requires careful navigation of numerous challenges. This viewpoint presents the authors' experiences and perspectives on the development and implementation of DHTTs, addressing both established practices and controversial topics. This article offers a practical guide organized into 10 recommendations derived from a multidisciplinary lecture series and associated workshop discussions on "Digital Health and Digital Biomarkers" held at the University of Luxembourg in 2023-2024. Key messages include the need to understand specific health care challenges, form interdisciplinary teams, incorporate patient feedback, select appropriate measurement technologies, ensure data integration and interoperability, apply advanced data science techniques, use scalable designs and open standards, comply with regulatory requirements, and maintain continuous evaluation and improvement. While the guide highlights essential practices, it also addresses contentious issues such as balancing innovation with regulatory compliance, addressing ethical concerns in artificial intelligence adoption, managing privacy versus the need for comprehensive data integration and open science, and managing the financial sustainability of DHTTs. The authors argue that digital health's greatest potential lies in its ability to provide participatory and personalized care, but this requires a delicate balance between technological advances and ethical, legal, and social implications. Overall, this workshop-derived viewpoint aims to help health care professionals, engineers, developers, and researchers not only adopt best practices but also address and resolve the controversial aspects inherent in the development of DHTTs.
Keywords: application; data interoperability; data privacy; digital health; electronic health record; health care technology; health monitoring; interdisciplinary collaboration; mobile health; real-time; recommendations; regulatory compliance; technology; telehealth; user engagement; user-centered design; wearable.
© Rebecca Ting Jiin Loo, Francesco Nasta, Mirco Macchi, Anaïs Baudot, Frada Burstein, Riley Bove, Maike Greve, Holger Fröhlich, Sara Khalid, Arne Küderle, Susan L Moore, Valerie Storms, John Torous, Enrico Glaab. Originally published in the Journal of Medical Internet Research (https://www.jmir.org).
Conflict of interest statement
Figures
References
-
- Kraus S, Schiavone F, Pluzhnikova A, Invernizzi AC. Digital transformation in healthcare: analyzing the current state-of-research. J Bus Res. 2021 Feb;123:557–567. doi: 10.1016/j.jbusres.2020.10.030. doi. - DOI
-
- Iqbal JD, Biller-Andorno N. The regulatory gap in digital health and alternative pathways to bridge it. Health Policy Technol. 2022 Sep;11(3):100663. doi: 10.1016/j.hlpt.2022.100663. doi. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

