Sustained Clinical Benefit of AAV Gene Therapy in Severe Hemophilia B
- PMID: 40499172
- PMCID: PMC7617823
- DOI: 10.1056/NEJMoa2414783
Sustained Clinical Benefit of AAV Gene Therapy in Severe Hemophilia B
Abstract
Background: Adeno-associated virus (AAV)-mediated gene therapy has emerged as a promising treatment for hemophilia B. Data on safety and durability from 13 years of follow-up in a cohort of patients who had been successfully treated with scAAV2/8-LP1-hFIXco gene therapy are now available.
Methods: Ten men with severe hemophilia B received a single intravenous infusion of the scAAV2/8-LP1-hFIXco vector in one of three dose groups (low-dose: 2×1011 vector genomes [vg] per kilogram of body weight [in two participants]; intermediate-dose: 6×1011 vg per kilogram [in two]; or high-dose: 2×1012 vg per kilogram [in six]). Efficacy outcomes included factor IX activity, the annualized bleeding rate, and factor IX concentrate use. Safety assessments included clinical events, liver function, and imaging.
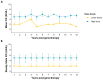
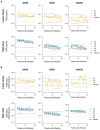
Results: Participants were followed for a median of 13.0 years (range, 11.1 to 13.8). Factor IX activity remained stable across the dose cohorts, with mean factor IX levels of 1.7 IU per deciliter in the low-dose group, 2.3 IU per deciliter in the intermediate-dose group, and 4.8 IU per deciliter in the high-dose group. Seven of the 10 participants did not receive prophylaxis. The median annualized bleeding rate decreased from 14.0 episodes (interquartile range, 12.0 to 21.5) to 1.5 episodes (interquartile range, 0.7 to 2.2), which represented a reduction by a factor of 9.7. Use of factor IX concentrate decreased by a factor of 12.4 (interquartile range, 2.2 to 27.1). A total of 15 vector-related adverse events occurred, primarily transient elevations in aminotransferase levels. Factor IX inhibitor, thrombosis, or chronic liver injury did not develop in any participant. Two cancers were identified but were deemed by the investigators, together with an expert multidisciplinary team, as being unrelated to the vector. A liver biopsy that was conducted in 1 participant 10 years after the infusion revealed transcriptionally active transgene expression in hepatocytes without fibrosis or dysplasia. Levels of neutralizing antibodies to AAV8 remained high throughout follow-up, thus indicating potential barriers to readministration of the vector.
Conclusions: A single administration of scAAV2/8-LP1-hFIXco gene therapy resulted in durable factor IX expression, sustained clinical benefit, and no late-onset safety concerns over a period of 13 years. These data support the long-term efficacy and safety of AAV gene therapy for severe hemophilia B. (Funded by the U.K. Medical Research Council and others; ClinicalTrials.gov number, NCT00979238; EudraCT number, 2005-005711-17.).
Copyright © 2025 Massachusetts Medical Society.
Figures
Comment in
-
AAV Gene Therapy in Severe Hemophilia B.N Engl J Med. 2025 Aug 21;393(8):829-830. doi: 10.1056/NEJMc2509703. N Engl J Med. 2025. PMID: 40834314 No abstract available.
-
AAV Gene Therapy in Severe Hemophilia B. Reply.N Engl J Med. 2025 Aug 21;393(8):830. doi: 10.1056/NEJMc2509703. N Engl J Med. 2025. PMID: 40834315 No abstract available.
References
-
- Nathwani AC, Rosales C, McIntosh J, et al. Long-term Safety and Efficacy Following Systemic Administration of a Self-complementary AAV Vector Encoding Human FIX Pseudotyped With Serotype 5 and 8 Capsid Proteins. Mol Ther. 2011;19(5):876–885.:mt2010274[pii] doi: 10.1038/mt.2010.274. - DOI - PMC - PubMed
-
- Pipe SW, Leebeek FWG, Recht M, et al. Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B. N Engl J Med. 2023;388(8):706–718. - PubMed
-
- Cuker A, Kavakli K, Frenzel L, et al. Gene Therapy with Fidanacogene Elaparvovec in Adults with Hemophilia B. N Engl J Med. 2024;391(12):1108–1118. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
