Regulatory Landscapes of Muscle Satellite Cells: From Mechanism to Application
- PMID: 40500132
- PMCID: PMC12394078
- DOI: 10.15283/ijsc25037
Regulatory Landscapes of Muscle Satellite Cells: From Mechanism to Application
Abstract
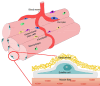
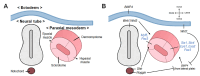
Muscle satellite cells (SCs), also known as muscle stem cells, are crucial for the regeneration, maintenance, and growth of skeletal muscles. SCs possess a distinctive capability to self-renew and differentiate, rendering them highly promising candidates for regenerative therapies and emerging cellular agriculture applications, including cultured meat production. This review explores the mechanisms that govern SC activation, proliferation, and commitment, and emphasizes their functional heterogeneity across anatomical regions. Region-specific gene expression, including that of homeobox (Hox) genes, contributes to the positional identity and myogenic potential. Understanding these regulatory landscapes is essential for optimizing SC expansion and improving their applications in muscle repair, stem cell-based therapies, and cellular manufacturing systems.
Keywords: Cell self renewal; Muscle development; Satellite cell; Skeletal muscle.
Conflict of interest statement
There is no potential conflict of interest to declare.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Myofibers cultured in viscoelastic hydrogels reveal the effects of integrin-binding and mechanosensing on muscle satellite cells.Acta Biomater. 2025 Jan 15;192:48-60. doi: 10.1016/j.actbio.2024.11.044. Epub 2024 Nov 28. Acta Biomater. 2025. PMID: 39615561 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Relationship between Pituitary Gland and Stem Cell in the Aspect of Hormone Production and Disease Prevention: A Narrative Review.Endocr Metab Immune Disord Drug Targets. 2025;25(7):509-526. doi: 10.2174/0118715303314551241031093717. Endocr Metab Immune Disord Drug Targets. 2025. PMID: 39812047 Review.
-
Extracellular vesicles derived from Schwann cells to enhance bone and dental tissue regeneration: a literature review.J Nanobiotechnology. 2025 Jul 11;23(1):502. doi: 10.1186/s12951-025-03585-7. J Nanobiotechnology. 2025. PMID: 40646600 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

