QSP modeling of loncastuximab tesirine with T-cell-dependent bispecific antibodies guides dose-regimen strategy
- PMID: 40500294
- PMCID: PMC12159156
- DOI: 10.1038/s41540-025-00544-8
QSP modeling of loncastuximab tesirine with T-cell-dependent bispecific antibodies guides dose-regimen strategy
Abstract
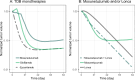
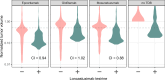
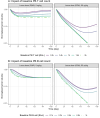
Antibody-drug conjugates (ADCs) and T-cell-dependent bispecific antibodies (TDBs) show single-agent efficacy in relapsed/refractory (R/R) lymphomas. While coadministering therapeutics with orthogonal mechanisms of action may safely enhance efficacy, testing every potential combination regimen is infeasible in the clinic. An integrated quantitative systems pharmacology model of a CD19-targeted ADC and CD3/CD20-targeted TDBs was developed to predict combination regimen efficacy in R/R diffuse large B-cell lymphoma (DLBCL). Clinically validated models of the ADC loncastuximab tesirine and TDB mosunetuzumab were combined and extended to additional TDBs (glofitamab and epcoritamab). Virtual DLBCL populations were calibrated using monotherapy response data, and tumor volume dynamics simulated under alternate combination dosing regimens and patient scenarios. Additive antitumor effects were predicted from the fourth cycle onward, with combination efficacy insensitive to loncastuximab tesirine dose reductions or patient lymphopenias. Results of the LOTIS-7 study (NCT04970901) will soon be available to assess these predictions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Y.L., A.K.W., J.D., T.K., and D.C.K. are employees of Metrum Research Group. M.T. is an employee and current equity holder at ADC Therapeutics. J.B. is an equity holder at ADC Therapeutics and was employed at ADC Therapeutics when the study was conducted.
Figures

References
-
- Scott, E. C. et al. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat. Rev. Drug Discov.22, 625–640 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

