Early life high fructose impairs microglial phagocytosis and neurodevelopment
- PMID: 40500435
- PMCID: PMC7617807
- DOI: 10.1038/s41586-025-09098-5
Early life high fructose impairs microglial phagocytosis and neurodevelopment
Abstract
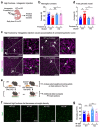
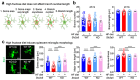
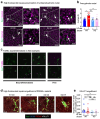
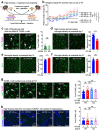
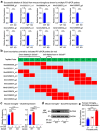
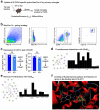
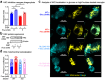
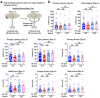
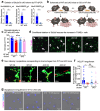
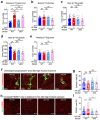
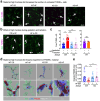
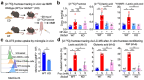
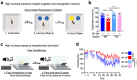
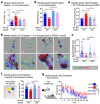
Despite the success of fructose as a low-cost food additive, epidemiological evidence suggests that high fructose consumption during pregnancy or adolescence is associated with disrupted neurodevelopment1-3. An essential step in appropriate mammalian neurodevelopment is the phagocytic elimination of newly formed neurons by microglia, the resident professional phagocyte of the central nervous system4. Whether high fructose consumption in early life affects microglial phagocytosis and whether this directly affects neurodevelopment remains unknown. Here we show that offspring born to female mice fed a high-fructose diet and neonates exposed to high fructose exhibit decreased phagocytic activity in vivo. Notably, deletion of the high-affinity fructose transporter GLUT5 (also known as SLC2A5) in neonatal microglia completely reversed microglia phagocytic dysfunction, suggesting that high fructose directly affects neonatal development by suppressing microglial phagocytosis. Mechanistically, we found that high-fructose treatment of mouse and human microglia suppresses phagocytosis capacity, which is rescued in GLUT5-deficient microglia. Additionally, we found that high fructose drives significant GLUT5-dependent fructose uptake and catabolism to fructose 6-phosphate, rewiring microglial metabolism towards a hypo-phagocytic state in part by enforcing mitochondrial localization of the enzyme hexokinase 2. Mice exposed to high fructose as neonates develop anxiety-like behaviour as adolescents-an effect that is rescued in GLUT5-deficient mice. Our findings provide a mechanistic explanation for the epidemiological observation that high-fructose exposure during early life is associated with increased prevalence of adolescent anxiety disorders.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: J.S.A.P. and K.R.K. are co-founders of Atish Technologies. K.R.K. serves on the scientific advisory board of NVision Imaging Technologies. J.S.A.P. and K.R.K. hold patents related to imaging and modulation of cellular metabolism. The other authors declare no competing interests.
Figures




References
Additional Refernces (cited only in “Methods”)
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

