SP140-RESIST pathway regulates interferon mRNA stability and antiviral immunity
- PMID: 40500448
- PMCID: PMC12310523
- DOI: 10.1038/s41586-025-09152-2
SP140-RESIST pathway regulates interferon mRNA stability and antiviral immunity
Abstract
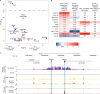
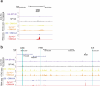
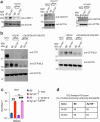
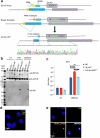
Type I interferons are essential for antiviral immunity1 but must be tightly regulated2. The conserved transcriptional repressor SP140 inhibits interferon-β (Ifnb1) expression through an unknown mechanism3,4. Here we report that SP140 does not directly repress Ifnb1 transcription. Instead, SP140 negatively regulates Ifnb1 mRNA stability by directly repressing the expression of a previously uncharacterized regulator that we call RESIST (regulated stimulator of interferon via stabilization of transcript; previously annotated as annexin 2 receptor). RESIST promotes Ifnb1 mRNA stability by counteracting Ifnb1 mRNA destabilization mediated by the tristetraprolin (TTP) family of RNA-binding proteins and the CCR4-NOT deadenylase complex. SP140 localizes within punctate structures called nuclear bodies that have important roles in silencing DNA-virus gene expression in the nucleus3. Consistent with this observation, we find that SP140 inhibits replication of the gammaherpesvirus MHV68. The antiviral activity of SP140 is independent of its ability to regulate Ifnb1. Our results establish dual antiviral and interferon regulatory functions for SP140. We propose that SP140 and RESIST participate in antiviral effector-triggered immunity5,6.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: R.E.V. is on the scientific advisory boards of Tempest Therapeutics and X-biotix.
Figures










Update of
-
The SP140-RESIST pathway regulates interferon mRNA stability and antiviral immunity.bioRxiv [Preprint]. 2025 Jan 29:2024.08.28.610186. doi: 10.1101/2024.08.28.610186. bioRxiv. 2025. Update in: Nature. 2025 Jul;643(8074):1372-1380. doi: 10.1038/s41586-025-09152-2. PMID: 39974928 Free PMC article. Updated. Preprint.
References
-
- Remick, B. C., Gaidt, M. M. & Vance, R. E. Effector-triggered immunity. Ann. Rev. Immunol.41, 453–481 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

