Multi-center randomized superiority clinical trial in the early phase of mechanically ventilated patients to preserve diaphragm thickness using non-invasive magnetic phrenic nerve stimulation: STIMIT ACTIVATOR 1 pivotal trial
- PMID: 40500715
- PMCID: PMC12153206
- DOI: 10.1186/s13063-025-08838-2
Multi-center randomized superiority clinical trial in the early phase of mechanically ventilated patients to preserve diaphragm thickness using non-invasive magnetic phrenic nerve stimulation: STIMIT ACTIVATOR 1 pivotal trial
Abstract
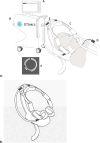
Background: Ventilator-induced diaphragmatic dysfunction (VIDD) occurs in up to 60% of mechanically ventilated patients and prolongs ventilatory dependance. The consequences of VIDD are muscle atrophy, reduction of strength, and injury of muscle fibers. Atrophy and contractile activity of the diaphragm can be estimated by ultrasound muscle thickness and thickening fraction. Prior experience demonstrates invasive electrical stimulation of the diaphragm helps preserve muscle thickness. This is the first study on a non-invasive phrenic nerve stimulator that aims to assess its feasibility, safety, and usefulness in preserving diaphragm thickness.
Methods: A multi-center randomized clinical trial will be performed in four intensive care units (ICUs) in the United States of America and Canada. Inclusion criteria include patients older than 21 years, in the first 48 h of mechanical ventilation (MV) and predicted to remain on the ventilator for at least 48 h. Patients with contraindications for phrenic nerve stimulation, severe chronic pulmonary diseases, or impossibility to measure diaphragm thickness with ultrasound will be excluded. Patients enrolled will be randomized to standard care (control) or 30-min daily non-invasive phrenic nerve stimulation up to 10 days after enrollment (intervention). The primary effectiveness endpoint is the change in diaphragm thickness on day 10, extubation, or death whichever occurs first. Secondary endpoints include change in diaphragm thickness on day 4, maximal inspiratory pressure before extubation, and time-to rapid shallow breathing index (RSBI) <105. Safety objectives include the proportion of device- or procedure-related adverse events (SAE). The estimated sample size will be 40 patients (20 per group). DISCUSSION: The STIMIT ACTIVATOR trial is a randomized multi-center study powered to elucidate whether non-invasive phrenic nerve stimulation is feasible, safe, and preserves diaphragm thickness. Meeting the primary endpoint will demonstrate its applicability in clinical practice to prevent diaphragmatic atrophy in ventilated patients.
Trial registration: ClinicalTrials.gov: NCT05883163, August 29, 2023.
Keywords: Critical care; Critical illness; Diaphragm; Mechanical ventilation; Phrenic nerve; Phrenic nerve stimulation; Ultrasonography; Ventilator-induced diaphragm dysfunction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate {24}: The protocol was already presented to the REB and approved in all sites with references. Copy of the original ethical approval documents is provided as an additional file (Additional file 2). Consent for publication {32}: A model consent document was distributed to local investigators at each center and is provided as an additional file (Additional file 3). Competing interests {28}: AP reports non-financial support from STIMIT AG (Biel, Switzerland) for travel. AP holds stocks in small amounts from BioNTech SE, Taiwan Semiconductor, Sony, Pfizer, Arcutis Biotherapeutics Inc, Sangamo Therapeutics, NIO, Ke Holdings; these holdings have not affected any decisions regarding this trial. SJS received grants from STIMIT AG (Biel, Switzerland) to support this trial. SJS reports grants and non-financial support from Reactive Robotics GmbH (Munich, Germany), ASP GmbH (Attendorn, Germany), STIMIT AG (Biel, Switzerland), ESICM (Geneva, Switzerland), grants, personal fees, and non-financial support from Fresenius Kabi Deutschland GmbH (Bad Homburg, Germany), grants from the Innovations fond of The Federal Joint Committee (G-BA), personal fees from Springer Verlag GmbH (Vienna, Austria) for educational purposes and Advanz Pharma GmbH (Bielefeld, Germany), non-financial support from national and international societies (and their congress organizers) in the field of anesthesiology and intensive care medicine, outside the submitted work. SJS holds stocks in small amounts from Alphabet Inc., Bayer AG, and Siemens AG; these holdings have not affected any decisions regarding his research or this trial.
Figures



References
-
- Dres M, Dube BP, Mayaux J, Delemazure J, Reuter D, Brochard L, et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. 2017;195(1):57–66. - PubMed
-
- Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183(3):364. - PubMed
-
- Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, et al. Diaphragm dysfunction on admission to the intensive care unit: Prevalence, risk factors, and prognostic impact - A prospective study. Am J Respir Crit Care Med. 2013;188(2):213–9. - PubMed
-
- Schepens T, Goligher EC. Lung- and diaphragm-protective ventilation in acute respiratory distress syndrome: rationale and challenges. Anesthesiology. 2019;130:620. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

