Laboratory-confirmed respiratory syncytial virus (RSV) hospitalizations: a national all ages cross-section evaluation, 2020-2024
- PMID: 40500807
- PMCID: PMC12153084
- DOI: 10.1186/s13584-025-00693-5
Laboratory-confirmed respiratory syncytial virus (RSV) hospitalizations: a national all ages cross-section evaluation, 2020-2024
Abstract
Background: New vaccines and monoclonal antibody (mAb) against respiratory syncytial virus (RSV) were recently approved for adults and infants, respectively. However, their inclusion in national vaccination programs has been slow. Accurate assessment of RSV disease burden among all ages is essential for the global introduction of these agents.
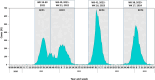
Methods: We evaluated all-ages burden of RSV hospitalizations, from 2020 to 2024, based on data collected by a new national laboratory-based hospital surveillance system. RSV-positive respiratory samples from patients hospitalized in general hospitals nationwide were reported. Data were analyzed by RSV circulation periods and age-group to determine hospitalization rates and 30-day mortality (30-DM) rates. We compared the laboratory-confirmed hospitalization rates with rates previously calculated based on ICD-9 codes.
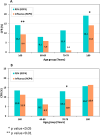
Results: RSV-confirmed hospitalizations were reported for all age-groups. The highest RSV hospitalization rates were found among patients < 1 year old. Patients ≥ 60 years old had the highest RSV hospitalization rates among ≥ 5 years old patients, and their 30-DM rates reached 14.7%, exceeding those of influenza. During the COVID-19 pandemic, lower rates of RSV-confirmed hospitalizations were reported among ≥ 60 years old patients, probably due to higher adherence to social distancing measures. We found higher numbers and rates of laboratory-confirmed hospitalizations among all age-groups ≥ 1 year old, than those previously reported by our group, based on ICD-9 codes.
Conclusions: Laboratory-confirmation of RSV is paramount for optimal assessment of RSV hospitalization burden, particularly beyond infancy, and for the global adoption of newly developed vaccines and mAb.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This evaluation was reviewed by the National Helsinki Committee for Human Medical Research of the Israel Ministry of Health; it was determined to be part of the Ministry of Health’s professional activity, which is presented in an anonymized and aggregated form, and as such, does not require an ethical committee’s approval or informed consent. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


References
-
- U.S. Food and Drug Administration. Respiratory Syncytial Virus (RSV). FDA has approved vaccines and monoclonal antibodies to protect against RSV. 2024. https://www.fda.gov/consumers/covid-19-flu-and-rsv/respiratory-syncytial... 2024 [updated 22 October 2024; cited 12 February 2025].
-
- European Medicines Agency. RSV. 2025. https://www.ema.europa.eu/en/search?search_api_fulltext=RSV&f%5B0%5D=ema... 2025 [updated 9 January 2025; cited 12 February 2025].
-
- Hammitt LL, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi SA, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386(9):837–46. - PubMed
-
- Wilkins D, Wählby-Hamrén U, Chang Y, Clegg LE, Domachowske J, Englund JA, et al. RSV neutralizing antibodies following nirsevimab and palivizumab dosing. Pediatrics. 2024;154:e2024067174. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

