Plasma microRNA Signature as Predictive Marker of Clinical Response to Therapy During Multiple Sclerosis
- PMID: 40501038
- PMCID: PMC12343307
- DOI: 10.1002/acn3.70093
Plasma microRNA Signature as Predictive Marker of Clinical Response to Therapy During Multiple Sclerosis
Abstract
Objective: Despite the availability of effective therapies for Multiple Sclerosis (MS), the unpredictable nature of disease progression and the variability in individual treatment outcomes call for reliable biomarkers. This pilot study aims to investigate the potential of plasma circulating microRNAs (miRNAs) as predictive biomarkers for clinical responses to dimethyl fumarate (DMF), a widely used oral treatment for MS.
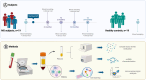
Methods: Peripheral blood samples were collected from nineteen treatment-naïve people with relapsing-remitting MS (pwRRMS) before and after 3, 6, 12, and 24 months of DMF administration, as well as from nineteen healthy individuals. MiRNAs were quantified by RT-qPCR after plasma RNA extraction, and peripheral blood immune cells were analyzed by flow cytometry. Pathway enrichment and protein-protein interaction analyses were performed to identify the biological processes and molecular networks associated with mRNAs targeted by the specific DMF-modulated miRNAs.
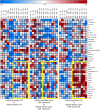
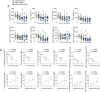
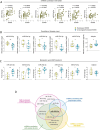
Results: We identified a DMF-modulated miRNA signature with significant changes occurring at early treatment stages. Notably, specific miRNAs were correlated with both clinical and immunological outcomes upon DMF treatment, including lymphocyte count reduction (let-7b-5p and miR-223-3p) and disease progression over 2 years (miR-223-3p, miR-23a-3p, miR-23b-3p, miR-27a-3p, and miR-27b-3p), suggesting their potential as predictive biomarkers for treatment response. Moreover, the validated mRNA targets of DMF-modulated miRNAs were enriched for IL-6 signaling and NRF2-dependent antioxidant pathways, highlighting the potential molecular mechanisms underpinning DMF efficacy.
Interpretation: This small exploratory study underscores the potential of plasma circulating miRNAs as candidate biomarkers for predicting therapeutic outcomes in MS and it calls for validation in larger studies, which may enhance our understanding of disease pathophysiology and offer a promising tool for personalized treatment strategies.
Keywords: circulating microRNA; dimethyl fumarate (DMF); multiple sclerosis.
© 2025 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
G.M. reports receiving research grant support from Merck, Biogen, and Novartis, and advisory board fees from Merck, Biogen, Novartis, and Roche. R.L. received personal compensation for speaking or consultancy from Biogen, Alexion, Sanofi, Merck, Bristol Myers Squibb, Janssen, Novartis, Amgen, and Roche. V.B.M. has received research grants from the Italian MS Society and Roche, and honoraria from Bayer, Biogen, Merck, Mylan, Novartis, Roche, Sanofi‐Genzyme, and Teva. S.B. reports receiving payment or honoraria for lectures, presentations, and speakers bureaus from Roche, Novartis, Biogen, Merck‐Serono, Alexion, Horizon, Janssen‐Cilag, BMS, Viatris, and support for attending meetings and/or travel from Roche, Novartis, Biogen, Merck‐Serono, Alexion, Horizon, Janssen‐Cilag, BMS, Viatris. G.A. reports receiving payment or honoraria for lectures, presentations, and speakers bureaus from Lundbeck. None of the other authors declare any conflicts of interest.
Figures


References
-
- Jakimovski D., Bittner S., Zivadinov R., et al., “Multiple Sclerosis,” Lancet 403 (2024): 183–202. - PubMed
-
- Filippi M., Bar‐Or A., Piehl F., et al., “Multiple Sclerosis,” Nature Reviews Disease Primers 4, no. 43 (2018). - PubMed
-
- Confavreux C., Vukusic S., Moreau T., and Adeleine P., “Relapses and Progression of Disability in Multiple Sclerosis,” New England Journal of Medicine 343 (2000): 1430–1438. - PubMed
MeSH terms
Substances
Grants and funding
- 2-SRA-2022-1192-S-B/JDRF/United States
- IT-BGT-11930/Biogen
- GR-2021-12373337/Italian Ministry of Health' : ' PNRR-MCNT2-2023-12378040
- PNRR-MAD-2022-12376126/Italian Ministry of Health' : ' PNRR-MCNT2-2023-12378040
- 2018/R/4/Fondazione Italiana Sclerosi Multipla
- 2022-PR-Single/013/Fondazione Italiana Sclerosi Multipla
- 20225KH7BZ/Italian Ministry of University and Research (MUR) ' : ' PE00000006
- 20228BRER5_002/Italian Ministry of University and Research (MUR) ' : ' PE00000006
- 2022LNHZAP/Italian Ministry of University and Research (MUR) ' : ' PE00000006
- 2022YMJXYT/Italian Ministry of University and Research (MUR) ' : ' PE00000006
- P2022CMK43/Italian Ministry of University and Research (MUR) ' : ' PE00000006
- P2022H8MZ4/Italian Ministry of University and Research (MUR) ' : ' PE00000006
- P2022T4PKT/Italian Ministry of University and Research (MUR) ' : ' PE00000006
LinkOut - more resources
Full Text Sources
Miscellaneous
