Disease-specific neuropathological alterations of the locus coeruleus in Alzheimer's disease, Down syndrome, and Parkinson's disease
- PMID: 40501099
- PMCID: PMC12159339
- DOI: 10.1002/alz.70262
Disease-specific neuropathological alterations of the locus coeruleus in Alzheimer's disease, Down syndrome, and Parkinson's disease
Abstract
Introduction: The locus coeruleus (LC), the brain's primary source of noradrenaline (NA), undergoes early neurodegeneration in Parkinson's disease (PD), Alzheimer's diseases (AD), and Down syndrome (DS); however, differences have not been examined in parallel.
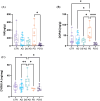
Methods: Post mortem brains (n = 67) from individuals with AD, DS-AD, and PD without and with dementia (PD-D) and controls were analyzed for amyloid beta (Aβ), phosphorylated tau (pTau), α-synuclein, endo-lysosomal alterations, biogenic amines, and selective biomarkers.
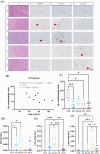
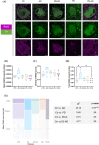
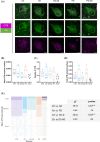
Results: LC degeneration correlated with age, peaking in AD and PD-D, while NA and dopaminergic metabolites were significantly reduced only in PD-D. DS-AD, the youngest group, showed the highest Aβ and pTau levels but the least noradrenergic neuron loss. We demonstrated for the first time that endosomal alterations were present in AD, lysosomal changes were present in PD-D/DS-AD, and DYRK1A, a key protein from chromosome 21, was elevated only in DS-AD.
Discussion: Loss of noradrenergic neurons may occur independently of amyloid and tau pathologies.
Highlights: We provide the first analysis of neuropathological and biochemical features including biogenic amines of the LC in AD, DS, and PD. Loss of noradrenergic neurons was most severe in AD and PD. Only in DS, levels of DYRK1A - a kinase encoded on chromosome 21 and implicated in neurodegenerative processes - were elevated and negatively correlated to biogenic amine levels. Although individuals with DS having AD were the youngest group, they had the highest levels of amyloid and tau pathologies, but less noradrenergic neurons loss compared to other disease groups.
Keywords: DYRK1A; endo‐lysosomal pathway; locus coeruleus; neurodegenerative diseases.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the Supporting Information.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

