Stimulants for disorders of consciousness in the intensive care unit: a randomized, placebo-controlled trial
- PMID: 40501148
- PMCID: PMC12493042
- DOI: 10.1093/brain/awaf228
Stimulants for disorders of consciousness in the intensive care unit: a randomized, placebo-controlled trial
Abstract
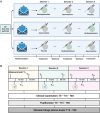
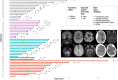
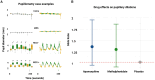
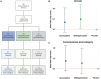
In the intensive care unit (ICU), management of unresponsive patients with brain injury focuses on preventing secondary brain damage. Therapeutic strategies that directly promote the recovery of consciousness are urgently needed. In an investigator-initiated, randomized, placebo-controlled, double-blind, cross-over trial, we studied the effects of apomorphine and methylphenidate in ICU patients with acute disorders of consciousness (DoC). We hypothesized that these stimulants would improve consciousness biomarkers assessed by automated pupillometry (primary outcome) and clinical signs of consciousness (secondary outcome). We randomized 50 ICU patients with DoC (14 female; mean age 63 ± 10 years; 48 with non-traumatic brain injuries) to strata consisting of three consecutive treatment sessions during which apomorphine, methylphenidate or placebo were administered. In total, we administered 112 study medications, including 36 doses of apomorphine, 39 doses of methylphenidate and 37 doses of placebo. Missing administrations were due to death, ICU discharge or spontaneous consciousness recovery. Plasma concentrations of stimulants confirmed drug exposure. We found no adverse events related to the trial drugs. Pupillometry recordings of sufficient quality (n = 590) were available from 48 (96%) patients. A pupillary response to a verbal arithmetic command (i.e. ≥3 pupillary dilations on five verbal arithmetic tasks) was identified during 70 (12%) of these recordings. Seven (15%) patients without any other observable response to spoken commands also passed a stricter threshold of ≥4 pupillary dilations, suggesting cognitive motor dissociation. Apomorphine [odds ratio (OR) 1.35, 95% confidence interval (CI): 0.93 to 1.96] and methylphenidate (OR 1.29, 95% CI: 0.89 to 1.86) did not significantly increase pupillary responses. However, after study drug administration, 10 (20%) patients showed improved clinical arousal at least once. Signs of arousal were noted after one dose of placebo, four doses of apomorphine (OR 5.04, 95% CI: 0.56 to 120.7) and seven doses of methylphenidate (OR 9.96, 95% CI: 1.36 to 235.8). Changes toward higher consciousness level categories were observed once after placebo, four times after apomorphine (OR 5.67, 95% CI 0.63 to 169.46) and three times after methylphenidate (OR 3.41, 95% CI 0.34 to 88.00). In a post hoc analysis, patients with greater pupillary responsiveness showed better arousal, suggesting that this condition may predict stimulant drug effects. In conclusion, while pupillometry revealed no direct drug effects on overall pupillary responses, stimulants may have triggered clinical arousal in some patients, particularly in those with greater pupillary responsiveness. These findings require replication but should guide future pharmacological trials aimed at improving consciousness recovery after brain injury.
Keywords: apomorphine; brain injury; clinical trial; coma; consciousness; methylphenidate; pupillometry.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- Gerges PRA, Moore L, Léger C, et al. Intensity of care and withdrawal of life-sustaining therapies in severe traumatic brain injury patients: A post-hoc analysis of a multicentre retrospective cohort study. Can J Anaesth. 2018;65:996–1003. - PubMed
-
- Kondziella D, Friberg CK, Frokjaer VG, Fabricius M, Møller K. Preserved consciousness in vegetative and minimal conscious states: Systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87:485–492. - PubMed
-
- Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019;380:2497–2505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

