Triplet-Triplet Annihilation Upconversion Is Impeded in Liposomes that Prevent Sensitizer and Annihilator Co-Confinement
- PMID: 40501149
- PMCID: PMC12207582
- DOI: 10.1021/acs.jpcb.5c01826
Triplet-Triplet Annihilation Upconversion Is Impeded in Liposomes that Prevent Sensitizer and Annihilator Co-Confinement
Abstract
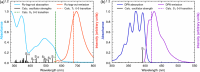
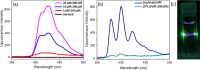
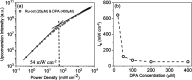
Triplet-triplet annihilation upconversion (TTA-UC) implemented in liposomes may be a promising tool in drug delivery and sensing. Indeed, we recently demonstrated that colocalization of lipophilic reagents to the membrane hydrophobic core improves the TTA-UC efficiency in liposomes compared to solution. Here, we examined if the counter is true, i.e., we evaluate if TTA-UC is inhibited when the sensitizer and annihilator occupy different regions within a single leaflet of a liposome membrane. To test this hypothesis, we used a Ru(II) complex, with tridentate ligand 2,6-di(quinolin-8-yl)pyridyl) (bqp) [Ru(bqp)(bpq-oct)]2+(Ru-bqp-oct) where oct is a C8 alkyl chain appended to facilitate integration into the liposome, as a sensitizer and diphenylanthracene (DPA) as an annihilator. TTA-UC from this pair was evaluated and compared in solution and liposomal nanovesicles. This Ru(II)-bqp complex was selected for its exceptionally long-lived emission and high triplet quantum yield, due to its expanded N-Ru-N bite angles. In solution, TTA-UC was efficient with a quantum yield of 3.11%, but in liposomes, no anti-Stokes shifted emission was observed even with an increased concentration of sensitizer and annihilator in the membrane. Molecular dynamics simulations were used to understand this effect and confirmed poor co-orientation of sensitizer and annihilator in the membrane was responsible for lack of TTA-UC in the membrane. DPA was determined to orient at the hydrophobic core, while the cationic Ru complex is embedded shallowly at the membrane interface, the closest approach of donor and acceptor in the membrane was determined as 0.7 nm. This work highlights the critical importance of colocalization of sensitizers and annihilators, even within a single membrane leaflet to facilitate Dexter energy transfer through collision in membrane-constrained TTA-UC systems and the value of MD simulations in system design.
Figures





References
-
- Baggaley E., Weinstein J. A., Williams J. A. G.. Lighting the Way to See inside the Live Cell with Luminescent Transition Metal Complexes. Coord. Chem. Rev. 2012;256(15–16):1762–1785. doi: 10.1016/j.ccr.2012.03.018. - DOI
-
- Arellano-Reyes R. A., Prabhakaran A., Sia R. C. E., Guthmuller J., Jha K. K., Yang T., Dietzek-Ivanšić B., McKee V., Keyes T. E.. BODIPY-Perylene Charge Transfer Compounds; Sensitizers for Triplet-Triplet Annihilation Up-conversion. Chem. - Eur. J. 2023;29(24):e202300239. doi: 10.1002/chem.202300239. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

