Omaveloxolone, But Not Dimethyl Fumarate, Improves Cardiac Function in Friedreich's Ataxia Mice With Severe Cardiomyopathy
- PMID: 40501183
- PMCID: PMC12229126
- DOI: 10.1161/JAHA.124.038505
Omaveloxolone, But Not Dimethyl Fumarate, Improves Cardiac Function in Friedreich's Ataxia Mice With Severe Cardiomyopathy
Abstract
Background: Friedreich's ataxia (FA) is a genetic disorder caused by a severe decrease in FXN (frataxin) protein expression in mitochondria. The clinical manifestation of this disorder is a cerebellar ataxia; however, the common lethal component in FA is cardiomyopathy.
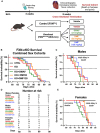
Methods: A conditional Fxnflox/null::MCK-Cre knockout (FXN-cKO) mouse model was used to mimic the late-stage severe cardiomyopathy in FA. Nrf2 (nuclear factor erythroid 2-related factor 2) inducers, omaveloxolone and dimethyl fumarate (DMF), were independently tested in this mouse model to determine the effects on cardiac health and lifespan.
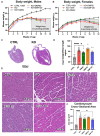
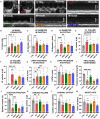
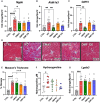
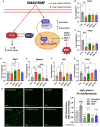
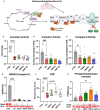
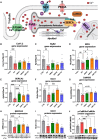
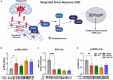
Results: Omaveloxolone significantly improved cardiac contractile function and markers of heart failure in FA such as Nppb, Aldh1a3, and Gdf15. Despite improvement in cardiac function, omaveloxolone did not prevent premature death in FXN-cKO animals and notably accelerated death in FXN-cKO females. Omaveloxolone decreased oxidative stress and inflammatory marker IL1β (interleukin-1 beta), and stimulated Nqo1 gene expression above control level. DMF restored elevated HO-1 (Hmox) expression and significantly increased Sirt1 expression. Although both omaveloxolone and DMF restored decreased SERCA2 (Atp2a) and MCU (Mcu) expression and ameliorated elevated phosphorylation of CaMKIIδ at Thr286 site in FA hearts, DMF did not improve cardiac contractile function and survival. Furthermore, neither omaveloxolone or DMF decreased hypertrophy and fibrosis (Masson trichrome staining and Lgals3 expression) or rescued impaired mitochondrial function and integrative stress response in FXN-cKO hearts.
Conclusions: These data demonstrate that omaveloxolone significantly improved contractile function but not survival in FA hearts because cardiac fibrosis and wall stress persisted even with omaveloxolone treatment. More studies are warranted to determine the cause of premature death in omaveloxolone-treated FXN-cKO female mice.
Keywords: Friedreich's ataxia; animal models of human disease; calcium signaling; cardiomyopathy; dimethyl fumarate; omaveloxolone.
Conflict of interest statement
None.
Figures
References
-
- Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

