Maternal plasma microRNAs as potential biomarkers for triaging pregnancies of unknown location and ectopic pregnancy diagnosis
- PMID: 40501483
- PMCID: PMC12156232
- DOI: 10.1016/j.ncrna.2025.05.005
Maternal plasma microRNAs as potential biomarkers for triaging pregnancies of unknown location and ectopic pregnancy diagnosis
Abstract
Background: Pregnancy of unknown location (PUL) is classified if an early pregnancy is not visualised on transvaginal ultrasonography (TVUS). Biomarkers currently used to triage PUL outcomes have varying accuracy. Delayed or missed diagnosis of ectopic pregnancies (EP) continue to cause significant morbidity and mortality. We investigated whether maternal plasma microRNAs (miRNAs) can predict and differentiate high-risk EP from viable (VIUP) or non-viable (NVIUP) intrauterine pregnancies.
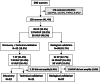
Methods: Plasma was collected from women with PUL/EP (n = 120), mostly between four to eight weeks' gestation, where outcomes of EP (n = 39), VIUP (n = 58) and NVIUP (miscarriage, n = 23) were determined using TVUS. Nanostring nCounter miRNA assay was used to examine the expression of ∼800 miRNAs in 22 women. Differentially expressed miRNAs were validated using RT-qPCR in 98 women.
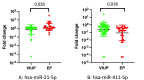
Results: Nanostring nCounter miRNA assay identified 19 miRNAs which were expressed significantly higher in EP/NVIUP compared with VIUP. Two miRNAs were validated in a second, separate validation cohort using RT-qPCR: hsa-miR-21-5p in EP was 2.8-fold higher than in VIUP (p = 0.03, ROC AUC = 0.64), and hsa-miR-411-5p had 0.2-fold decreased expression (p = 0.02, ROC AUC = 0.66). Combining the divergent miRNAs as a ratio improved discrimination of EP from VIUP (p < 0.001, ROC AUC = 0.74).
Conclusion: Plasma miRNAs are differentially expressed in EP and VIUP and are detectable as early as four gestational weeks. Exploring miRNA targets may further understanding of EP pathophysiology, offering the potential to use miRNA as predictive and diagnostic markers in early pregnancy.
Keywords: Biomarkers; Ectopic pregnancy; MicroRNA; PUL; Plasma.
© 2025 The Authors.
Conflict of interest statement
The findings of this study have been filed and published under the remit of two patent applications: 11,075 (microRNA discovery work – GB 2113344·2) and 10,439 (microRNA biological validation work – PCT/GB2020/050,324). The authors have declared that no other conflict of interest exists.
Figures





References
-
- Banerjee S., Aslam N., Woelfer B., Lawrence A., Elson J., Jurkovic D. Expectant management of early pregnancies of unknown location: a prospective evaluation of methods to predict spontaneous resolution of pregnancy. BJOG. 2001;108(2):158–163. - PubMed
-
- Mol B.W., Hajenius P.J., Engelsbel S., Ankum W.M., Van der Veen F., Hemrika D.J., et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil. Steril. 1998;70(5):972–981. - PubMed
-
- Kirk E., Condous G., Van Calster B., Van Huffel S., Timmerman D., Bourne T. Rationalizing the follow-up of pregnancies of unknown location. Hum. Reprod. 2007;22(6):1744–1750. - PubMed
-
- Cordina M., Schramm-Gajraj K., Ross J.A., Lautman K., Jurkovic D. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118(6):693–697. - PubMed
LinkOut - more resources
Full Text Sources

