A versatile route towards 6-arylpipecolic acids
- PMID: 40501506
- PMCID: PMC12152316
- DOI: 10.3762/bjoc.21.88
A versatile route towards 6-arylpipecolic acids
Abstract
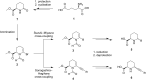
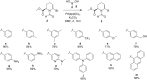
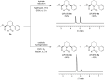
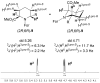
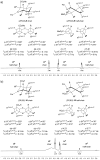
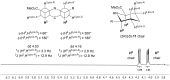
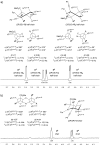
Pipecolic acid is known as a non-proteinogenic amino acid with a secondary amine. It contains a six-membered ring and is, like its five-membered correlate, known for its secondary structure inducing properties, which are particularly useful in the design of peptide conformations. We present a new and improved way to generate enantiomerically pure pipecolic acid derivatives with aryl modifications in C6 position by utilising the chiral pool of a non-proteinogenic amino acid in combination with transition metal-catalysed cross-coupling reactions. Moreover, we present an in-depth NMR analysis of the key intermediate steps, which illustrates the conformational constraints in accordance with coupling constants and resulting dihedral angles.
Keywords: Suzuki–Miyaura cross-coupling; conformational restraints; dihedral angle NMR; half-chair conformation; modified amino acids; pipecolic acid; stereoselective hydrogenation.
Copyright © 2025, Gebel et al.
Figures
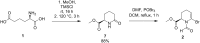

References
LinkOut - more resources
Full Text Sources
