This is a preprint.
Ketogenesis is dispensable for the metabolic adaptations to caloric restriction
- PMID: 40501543
- PMCID: PMC12154805
- DOI: 10.1101/2025.05.29.656153
Ketogenesis is dispensable for the metabolic adaptations to caloric restriction
Abstract
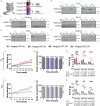
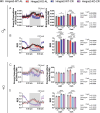
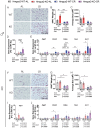
Caloric restriction (CR) robustly extends the health and lifespan of diverse species. When fed once daily, CR-treated mice rapidly consume their food and endure a prolonged fast between meals. As fasting is associated with a rise in circulating ketones, we decided to investigate the role of ketogenesis in CR using mice with whole-body ablation of Hmgcs2, the rate-limiting enzyme producing the main ketone body β-hydroxybutyrate (βHB). Here, we report that Hmgcs2 is largely dispensable for many metabolic benefits of CR, including CR-driven changes in adiposity, glycemic control, liver autophagy, and energy balance. Although we observed sex-specific effects of Hmgcs2 on insulin sensitivity, fuel selection, and adipocyte gene expression, the overall physiological response to CR remains robust in mice lacking Hmgcs2. To gain insight into why deletion of Hmgcs2 does not disrupt CR, we measured fasting βHB levels as mice began a CR diet. Surprisingly, as CR-fed mice adapt to CR, they no longer engage high levels of ketogenesis during the daily fast. Our work suggests that the benefits of long-term CR in mice are not mediated by ketogenesis.
Keywords: BHB; caloric restriction; dietary restriction; ketogenesis; ketones; metabolic health; metabolism.
Conflict of interest statement
DWL has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases.
Figures




Similar articles
-
Fasting is required for many of the benefits of calorie restriction in the 3xTg mouse model of Alzheimer's disease.bioRxiv [Preprint]. 2024 Sep 24:2024.09.19.613904. doi: 10.1101/2024.09.19.613904. bioRxiv. 2024. Update in: Nat Commun. 2025 Aug 4;16(1):7147. doi: 10.1038/s41467-025-62416-3. PMID: 39386545 Free PMC article. Updated. Preprint.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Oxycodone for cancer-related pain.Cochrane Database Syst Rev. 2022 Jun 9;6(6):CD003870. doi: 10.1002/14651858.CD003870.pub7. Cochrane Database Syst Rev. 2022. PMID: 35679121 Free PMC article.
-
Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta-analysis.Cochrane Database Syst Rev. 2021 Nov 16;11(11):CD012775. doi: 10.1002/14651858.CD012775.pub2. Cochrane Database Syst Rev. 2021. PMID: 34784425 Free PMC article.
-
Citrullinemia Type I.2004 Jul 7 [updated 2022 Aug 18]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2004 Jul 7 [updated 2022 Aug 18]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301631 Free Books & Documents. Review.
References
-
- Pak H.H., Haws S.A., Green C.L., Koller M., Lavarias M.T., Richardson N.E., Yang S.E., Dumas S.N., Sonsalla M., Bray L., et al. (2021). Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat Metab 3, 1327–1341. 10.1038/s42255-021-00466-9. - DOI - PMC - PubMed
Publication types
Grants and funding
- R56 AG056771/AG/NIA NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- F31 AG082504/AG/NIA NIH HHS/United States
- R01 AG062328/AG/NIA NIH HHS/United States
- F32 AG077916/AG/NIA NIH HHS/United States
- RF1 AG056771/AG/NIA NIH HHS/United States
- R01 DK125859/DK/NIDDK NIH HHS/United States
- K99 AG084921/AG/NIA NIH HHS/United States
- K00 AG083290/AG/NIA NIH HHS/United States
- U01 AG081482/AG/NIA NIH HHS/United States
- F31 AG081115/AG/NIA NIH HHS/United States
- IS1 BX005524/BX/BLRD VA/United States
- R01 AG056771/AG/NIA NIH HHS/United States
- F99 AG083290/AG/NIA NIH HHS/United States
- R01 AG084156/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
