This is a preprint.
Intranasal Dantrolene Nanoparticles for Treatment of Amyotrophic Lateral Sclerosis as a Disease-Modifying Drug
- PMID: 40501612
- PMCID: PMC12154592
- DOI: 10.1101/2025.05.21.655232
Intranasal Dantrolene Nanoparticles for Treatment of Amyotrophic Lateral Sclerosis as a Disease-Modifying Drug
Abstract
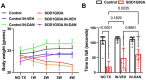
Calcium dysregulation, caused by pathological activation of ryanodine receptors, contributes to motor neuron degeneration, motor dysfunction, and muscle weakness in SOD1-G93A transgenic amyotrophic lateral sclerosis (ALS) mice. This study investigates the therapeutic efficacy of intranasally administered dantrolene nanoparticles, a ryanodine receptor antagonist, on motor neuron function, muscle strength, spinal cord degeneration, and survival outcomes. Male and female C57BL/6SJLF1 non-transgenic control and SOD1-G93A ALS transgenic mice were assigned to one of three experimental groups: 1) NO TX: No treatment control; 2) IN-DAN: Intranasal administration of dantrolene in the Ryanodex formulation vehicle (RFV), at a dosage of 5mg/kg, administered daily from ages 90-120 days; 3) IN-VEH: Intranasal administration of RFV alone (as a vehicle control), following the same dosing schedule as the IN-DAN condition. Body weight and general motor function were monitored weekly, with survival recorded daily throughout the treatment period. At the treatment conclusion, neurological function was comprehensively evaluated using a standardized neurological scoring system. Motor coordination and balance were assessed using the balance beam test (beam widths of 12 mm and 6 mm) and the rotarod test. Muscle strength was evaluated by measuring grip force using the Kondziela inverted screen test. After behavioral testing, spinal cord tissues were collected for analysis. The levels of neurofilament light chain (NFL), a skeletal neuron protein, and spinal cord weight were determined to measure spinal cord degeneration. Compared to non-transgenic control mice, SOD1-G93A mice exhibited significantly elevated neurological scores, indicating severely impaired neurological function. This deterioration was robustly and significantly ameliorated by IN-DAN treatment by 90% (P<0.0001). Similarly, ALS mice demonstrated impairments in motor coordination and balance on the beam balance test, with dramatic and significant increases in crossing time and the number of foot slips. These impairments were greatly and significantly mitigated by IN-DAN treatment, by 78% in crossing time (P<0.0001) and 84% in the number of slips (P<0.0001) on the 12 mm-wide beam, but not by the vehicle control. ALS mice demonstrated progressive body weight loss as well, which was similarly reversed by IN-DAN treatment, but not by the vehicle control. Muscle strength, as measured by grip force, was significantly reduced in ALS mice but robustly preserved IN-DAN treatment, which prevented the decrease by 213% (P<0.0001), while the vehicle control had no effect. Spinal cord weight was significantly reduced in ALS mice, a trend reversed by intranasal dantrolene nanoparticle treatment, but not by the vehicle control. Survival analysis revealed that 100% of control mice survived through the 30-day treatment period (up to 120 days of age), while survival in untreated or vehicle-treated ALS mice dropped to 67%. In contrast, ALS mice treated with intranasal dantrolene nanoparticles demonstrated a significantly improved survival rate of 89%. Thus, intranasal dantrolene nanoparticle treatment significantly and robustly improved neurological outcomes in SOD1-G93A ALS mice, inhibiting neurological impairment, motor dysfunction, balance deficits, and muscle weakness. These improvements were associated with a marked inhibition of spinal cord weight loss. Additionally, dantrolene treatment reversed body weight loss and significantly improved survival probability in ALS mice.
Conflict of interest statement
Conflict of Interest Huafeng Wei is an inventor for USA patents application by the University of Pennsylvania repurposing intranasal dantrolene nanoparticles to treat neurodegenerative diseases.
Figures




References
-
- Gonzalez-Sanchez M., Ramirez-Exposito M. J. & Martinez-Martos J. M. Pathophysiology, Clinical Heterogeneity, and Therapeutic Advances in Amyotrophic Lateral Sclerosis: A Comprehensive Review of Molecular Mechanisms, Diagnostic Challenges, and Multidisciplinary Management Strategies. Life (Basel) 15, doi: 10.3390/life15040647 (2025). - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
