This is a preprint.
Instant fluorescence lifetime imaging microscopy reveals mechano-metabolic reprogramming of stromal cells in breast cancer peritumoral microenvironments
- PMID: 40501613
- PMCID: PMC12154642
- DOI: 10.1101/2025.05.28.656717
Instant fluorescence lifetime imaging microscopy reveals mechano-metabolic reprogramming of stromal cells in breast cancer peritumoral microenvironments
Abstract
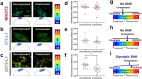
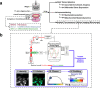
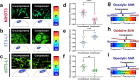
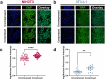
The breast peritumor microenvironment (pTME) is increasingly recognized as a mediator of breast cancer progression and treatment resistance. However, if and how growth-induced tumor compressive forces (i.e., solid stresses) influence the breast pTME remains unclear. Here we show using instant fluorescence lifetime imaging microscopy (FLIM)-a frequency-domain FLIM system capable of simultaneous image acquisition and instantaneous data processing-that breast tumor-mimicking in vitro compression promotes metabolic changes in stromal cells found in the breast pTME. Namely, compression shifts NIH3T3 fibroblasts and differentiated 3T3-L1 (d3T3-L1) adipocytes toward a more glycolytic state, while it promotes increased oxidative phosphorylation in 3T3-L1 undifferentiated adipocytes. The gold-standard Seahorse extracellular flux assay fails to capture these changes, underscoring the superior sensitivity of instant FLIM in detecting metabolic shifts. We validate these phenotypic findings at the transcriptomic level via RNA sequencing, confirming that compressed fibroblasts downregulate oxidative phosphorylation and upregulate glycolysis compared to uncompressed controls. We further demonstrate that compression induces mitochondrial dysregulation in undifferentiated adipocytes, driven in part by upregulated mitophagy and disrupted fusion dynamics. Finally, we confirm that these stromal cell types recapitulate these distinct metabolic states in human breast cancer patient samples, consistent with our in vitro findings. By elucidating mechano-metabolic interactions occurring at the tumor-host interface, these results will inform the development of innovative mechano-metabolic reprogramming treatment strategies to improve breast cancer patient survival.
Keywords: glycolysis; host tissue; mitochondria; oxidative phosphorylation; solid stress.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures



Similar articles
-
Strategies to improve smoking cessation rates in primary care.Cochrane Database Syst Rev. 2021 Sep 6;9(9):CD011556. doi: 10.1002/14651858.CD011556.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693994 Free PMC article.
-
Interventions for promoting habitual exercise in people living with and beyond cancer.Cochrane Database Syst Rev. 2018 Sep 19;9(9):CD010192. doi: 10.1002/14651858.CD010192.pub3. Cochrane Database Syst Rev. 2018. PMID: 30229557 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
