This is a preprint.
c-Myc inhibits macrophage antimycobacterial response in Mycobacterium tuberculosis infection
- PMID: 40501729
- PMCID: PMC12157703
- DOI: 10.1101/2025.01.09.632095
c-Myc inhibits macrophage antimycobacterial response in Mycobacterium tuberculosis infection
Update in
-
c-Myc Inhibits Macrophage Antimycobacterial Response in Mycobacterium tuberculosis Infection.J Infect Dis. 2025 Oct 15;232(4):e691-e703. doi: 10.1093/infdis/jiaf456. J Infect Dis. 2025. PMID: 40884499 Free PMC article.
Abstract
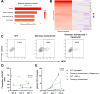
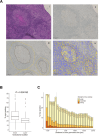
Mycobacterium tuberculosis (MTB) is a major global cause of mortality worldwide, responsible for over a million deaths annually. Despite this burden, natural immunity prevents disease in more than 90% of exposed individuals. Previous studies have identified interferon-gamma (IFN-γ) as a key regulator of innate immune defense against MTB. Here, we investigate the impact of IFN-γ timing on macrophage-mediated control of MTB infection. We demonstrate that IFN-γ exposure before infection enhances macrophage antibacterial activity, whereas post-infection exposure does not. Further investigation into this phenotype revealed a strong association between c-Myc signaling and macrophage function in MTB control, as identified using unbiased in vitro systems approaches. Given the challenge of perturbing c-Myc in primary cells, we developed a lentiviral system for c-Myc inhibition and overexpression. Using a tetracycline-inducible Omomyc system - a small peptide inhibitor of c-Myc - we show that c-Myc inhibition promotes a pro-inflammatory macrophage phenotype with enhanced antimycobacterial activity. Mechanistically, c-Myc inhibition induces metabolic reprogramming via increased mTORC1 activity, leading to upregulated inducible nitric oxide synthase and improved bacterial control. In vivo analyses, including murine models and human clinical histopathology, reveal a strong correlation between c-Myc expression and MTB persistence, as well as active tuberculosis (TB), suggesting a role for c-Myc in immune evasion. These findings reveal c-Myc as a potential mediator of immune privilege in MTB infection and highlight its role as a promising target for novel TB therapies aimed at enhancing macrophage function.
Figures




References
-
- WHO. Global Tuberculosis Report. Geneva, Switzerland: World Health Organization. (2024).
-
- Cohen S. B., Gern B. H. & Urdahl K. B. The Tuberculous Granuloma and Preexisting Immunity. Annu Rev Immunol 40, 589–614 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
