This is a preprint.
Microglial SWELL1 Deficiency Drives Male-Specific Seizure Vulnerability but Paradoxical Neuroprotection through Impaired Phagocytosis
- PMID: 40501741
- PMCID: PMC12154700
- DOI: 10.1101/2025.05.26.656163
Microglial SWELL1 Deficiency Drives Male-Specific Seizure Vulnerability but Paradoxical Neuroprotection through Impaired Phagocytosis
Abstract
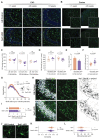
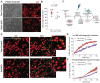
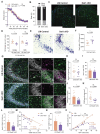
The discovery of genes encoding the volume-regulated anion channel (VRAC) has enabled detailed exploration of its cell type-specific roles in the brain. LRRC8A (SWELL1) is the essential VRAC subunit. We observed seizure-induced, subunit-specific changes in microglial VRAC expression and investigated its function using conditional knockout (cKO) of LRRC8A in microglia. SWELL1 cKO mice exhibited a male-specific increase in kainate-induced seizure severity yet showed paradoxical neuroprotection against seizure-associated neuronal loss. Mechanistically, SWELL1 deletion led to a cell-autonomous reduction in microglial density and decreased release of VRAC-permeable neuroactive metabolites, including taurine, GABA, and glutamate in culture. Additionally, impaired phagocytic kinetics and reduced lysosomal biogenesis contributed to the observed neuroprotection. These findings reveal novel roles for microglial VRAC in regulating seizure outcomes and microglia-neuron interactions.
Keywords: Epilepsy; SWELL1; VRAC; microglia; neuroprotection; phagocytosis; seizures; sex-differences.
Conflict of interest statement
Competing interests The authors declare no competing interests in relation to this work.
Figures



References
-
- Dardiotis E., Siokas V., Pantazi E., Dardioti M., Rikos D., Xiromerisiou G., Markou A., Papadimitriou D., Speletas M., Hadjigeorgiou G. M., A novel mutation in TREM2 gene causing Nasu-Hakola disease and review of the literature. Neurobiology of Aging 53, (2017). - PubMed
-
- Papapetropoulos S., Pontius A., Finger E., Karrenbauer V., Lynch D. S., Brennan M., Zappia S., Koehler W., Schoels L., Hayer S. N., Konno T., Ikeuchi T., Lund T., Orthmann-Murphy J., Eichler F., Wszolek Z. K., Adult-Onset Leukoencephalopathy With Axonal Spheroids and Pigmented Glia: Review of Clinical Manifestations as Foundations for Therapeutic Development. Frontiers in Neurology 12, (2022). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
