This is a preprint.
PTBP1 Depletion in Mature Astrocytes Reveals Distinct Splicing Alterations Without Neuronal Features
- PMID: 40501814
- PMCID: PMC12157468
- DOI: 10.1101/2025.05.30.657115
PTBP1 Depletion in Mature Astrocytes Reveals Distinct Splicing Alterations Without Neuronal Features
Abstract
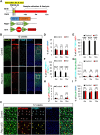
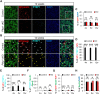
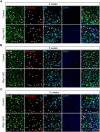
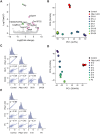
Astrocyte-to-neuron reprogramming via depletion of PTBP1, a potent repressor of neuronal splicing, has been proposed as a therapeutic strategy, but its efficacy remains debated. While some reported successful conversion, others disputed this, citing a lack of neuronal gene expression as evidence of failed reprogramming. This interpretation was further challenged, attributed to incomplete PTBP1 inactivation, fueling ongoing controversy. Mechanistic understanding of the conversion, or the lack thereof, requires investigating, in conjunction with lineage tracing, the effect of Ptbp1 loss of function in mature astrocytes on RNA splicing, which has not yet been examined. Here, we genetically ablated PTBP1 in adult Aldh1l1-Cre/ERT2 Ai14 mice to determine whether lineage traced Ptbp1 knockout astrocytes exhibited RNA splicing alterations congruent with neuronal differentiation. We found no widespread induction of neurons, despite a minuscule fraction of knockout cells showing neuron-like transcriptomic signatures. Importantly, PTBP1 loss in mature astrocytes induced splicing alterations unlike neuronal splicing patterns. These findings suggest that targeting PTBP1 alone is ineffective to drive neuronal reprogramming and highlight the need for combining splicing and lineage analyses. Loss of astrocytic PTBP1 is insufficient to induce neuronal splicing, contrasting with its well-known role in other non-neuronal cells, and instead affects a distinct astrocytic splicing program.
Figures


Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Ptbp1 is not required for retinal neurogenesis and cell fate specification.bioRxiv [Preprint]. 2025 Jul 3:2025.07.02.662808. doi: 10.1101/2025.07.02.662808. bioRxiv. 2025. PMID: 40631124 Free PMC article. Preprint.
-
Single-incision sling operations for urinary incontinence in women.Cochrane Database Syst Rev. 2017 Jul 26;7(7):CD008709. doi: 10.1002/14651858.CD008709.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Oct 27;10:CD008709. doi: 10.1002/14651858.CD008709.pub4. PMID: 28746980 Free PMC article. Updated.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Bocchi R., Masserdotti G. & Götz M. Direct neuronal reprogramming: Fast forward from new concepts toward therapeutic approaches. Neuron 110, 366–393 (2022). - PubMed
-
- Yang Y. et al. Enhanced Rejuvenation in Induced Pluripotent Stem Cell-Derived Neurons Compared with Directly Converted Neurons from an Aged Mouse. Stem Cells Dev. 24, 2767–2777 (2015). - PubMed
-
- Matsuda T. et al. Pioneer Factor NeuroD1 Rearranges Transcriptional and Epigenetic Profiles to Execute Microglia-Neuron Conversion. Neuron 101, 472–485.e7 (2019). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
