This is a preprint.
TRPV4 modulates substrate stiffness mechanosensing and transcellular pore formation in human Schlemm's canal cells
- PMID: 40501826
- PMCID: PMC12154856
- DOI: 10.1101/2025.05.25.656000
TRPV4 modulates substrate stiffness mechanosensing and transcellular pore formation in human Schlemm's canal cells
Abstract
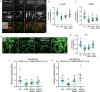
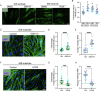
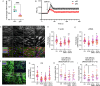
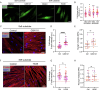
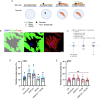
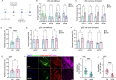
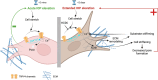
Pathological changes in the biomechanical environment of Schlemm's canal (SC) inner wall cells, such as substrate stiffening and increased cellular stretch, are associated with ocular hypertension, a key risk factor for the development of glaucoma. Cell membrane stretch can trigger the activation of transient receptor potential vanilloid 4 (TRPV4) mechanosensitive ion channels, allowing calcium influx and initiating downstream signaling. However, the precise role of TRPV4 in SC cell mechanobiology remains unclear. Here, we demonstrate that sustained inhibition of TRPV4 activity modulates substrate stiffness mechanosensing to thereby affect the remodeling of the actin cytoskeleton and extracellular matrix of SC cells. This is accompanied by a reduction in cell stiffness and an increase in transcellular pore forming ability, potentially lowing outflow resistance and risk of ocular hypertension. Conversely, acute activation of TRPV4 channels induces Ca2+ influx, increasing transcellular pore formation in SC cells. Notaly, reduced TRPV4 mechanosensing was observed in glaucomatous SC cells, resulting in reduced transcellular pore forming ability. These findings suggest novel potential strategies based on targeting TRPV4 in SC cells for the treatment of ocular hypertension in glaucoma.
Keywords: ECM stiffening; Glaucoma; calcium signaling; hydrogel; ion channel; mechanobiology; tissue engineering; trancellular pore.
Conflict of interest statement
Disclosure The authors report no conflicts of interest.
Figures
Similar articles
-
Trpv4-mediated mechanotransduction regulates the differentiation of valvular interstitial cells to myofibroblasts: implications for aortic valve stenosis.Am J Physiol Cell Physiol. 2025 May 1;328(5):C1558-C1570. doi: 10.1152/ajpcell.00977.2024. Epub 2025 Apr 9. Am J Physiol Cell Physiol. 2025. PMID: 40203884 Free PMC article.
-
Targeted Delivery of Cell Softening Micelles to Schlemm's Canal Endothelial Cells for Treatment of Glaucoma.Small. 2020 Oct;16(43):e2004205. doi: 10.1002/smll.202004205. Epub 2020 Oct 4. Small. 2020. PMID: 33015961 Free PMC article.
-
Rho kinase inhibitor for primary open-angle glaucoma and ocular hypertension.Cochrane Database Syst Rev. 2022 Jun 10;6(6):CD013817. doi: 10.1002/14651858.CD013817.pub2. Cochrane Database Syst Rev. 2022. PMID: 35686679 Free PMC article.
-
TRPV4 activation by TGFβ2 enhances cellular contractility and drives ocular hypertension.Elife. 2025 Jun 24;14:RP104894. doi: 10.7554/eLife.104894. Elife. 2025. PMID: 40552711 Free PMC article.
-
Perioperative medications for preventing temporarily increased intraocular pressure after laser trabeculoplasty.Cochrane Database Syst Rev. 2017 Feb 23;2(2):CD010746. doi: 10.1002/14651858.CD010746.pub2. Cochrane Database Syst Rev. 2017. PMID: 28231380 Free PMC article.
References
-
- Brubaker R.F. (1991). Flow of aqueous humor in humans [The Friedenwald Lecture]. Invest Ophthalmol Vis Sci 32, 3145–3166. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
