This is a preprint.
Remote automated delivery of mechanical stimuli coupled to brain recordings in behaving mice
- PMID: 40501830
- PMCID: PMC12157349
- DOI: 10.1101/2024.05.06.592101
Remote automated delivery of mechanical stimuli coupled to brain recordings in behaving mice
Update in
-
Remote automated delivery of mechanical stimuli coupled to brain recordings in behaving mice.Elife. 2025 Oct 17;13:RP99614. doi: 10.7554/eLife.99614. Elife. 2025. PMID: 41104746 Free PMC article.
Abstract
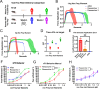
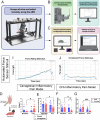
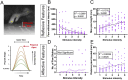
The canonical framework for testing pain and mechanical sensitivity in rodents is manual delivery of stimuli to the paw. However, this approach is time consuming, produces variability in results, requires significant training, and is ergonomically unfavorable to the experimenter. To circumvent limitations in manual delivery of stimuli, we have created a device called the ARM (Automated Reproducible Mechano-stimulator). Built using a series of linear stages, cameras, and stimulus holders, the ARM is more accurate at hitting the desired target, delivers stimuli faster, and decreases variability in delivery of von Frey hair filaments. We demonstrate that the ARM can be combined with traditional measurements of pain behavior and automated machine-learning based pipelines. Importantly, the ARM enables remote testing of mice with experimenters outside the testing room. Using remote testing, we found that mice habituated more quickly when an experimenter was not present and experimenter presence leads to significant sex-dependent differences in paw withdrawal and pain associated behaviors. Lastly, to demonstrate the utility of the ARM for neural circuit dissection of pain mechanisms, we combined the ARM with cellular-resolved microendoscopy in the amygdala, linking stimulus, behavior, and brain activity of amygdala neurons that encode negative pain states. Taken together, the ARM improves speed, accuracy, and robustness of mechanical pain assays and can be combined with automated pain detection systems and brain recordings to map central control of pain.
Figures



References
-
- Israel H., Neeb L., & Reuter U. (2018). CGRP monoclonal antibodies for the preventative treatment of migraine. Current pain and headache reports, 22, 1–6. - PubMed
-
- Chesler E. J., Wilson S. G., Lariviere W. R., Rodriguez-Zas S. L., & Mogil J. S. (2002). Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neuroscience & Biobehavioral Reviews, 26(8), 907–923. - PubMed
-
- Sorge R. E., Martin L. J., Isbester K. A., Sotocinal S. G., Rosen S., Tuttle A. H., Wieskopf J. S., Acland E. L., Dokova A., Kadoura B., Leger P., Mapplebeck J. C. S., McPhail M., Delaney A., Wigerblad G. Schumann A. P., Quinn T., Frasnelli J., Svensson C. I.,…& Mogil J. S. (2014). Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nature methods, 11(6), 629–632. - PubMed
-
- Becker L. J., Fillinger C., Waegaert R., Journée S. H., Hener P., Ayazgok B., Humo M., Karatas M., Thouaye M., Gaikwad M., Degiorgis L., Santin M. D. N., Mondino M., Barrot M., Ibrahim E. C., Turecki G., Belzeaux R., Veinante P., Harsan L. A.,…& Yalcin I. (2023). The basolateral amygdala-anterior cingulate pathway contributes to depression-like behaviors and comorbidity with chronic pain behaviors in male mice. Nature Communications, 14(1), 2198. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
