This is a preprint.
The Neurofibrillary Tangle Maturity Scale: A Novel Framework for Tangle Pathology Evaluation in Alzheimer's Disease
- PMID: 40501844
- PMCID: PMC12157467
- DOI: 10.1101/2025.06.02.657435
The Neurofibrillary Tangle Maturity Scale: A Novel Framework for Tangle Pathology Evaluation in Alzheimer's Disease
Abstract
Neurofibrillary tangles are dynamic neuropathologic hallmarks of Alzheimer's disease with a hypothesized lifespan morphologically-defined by three maturity levels: pretangles, mature tangles, and ghost tangles. To better understand the progression of tangle pathophysiology, we characterized tangle maturity level predilection of 15 tau antibodies recognizing a broad range of linear, phosphorylation, conformational, and truncation epitopes in the hippocampus of 24 postmortem brains. We developed the tangle maturity scoring system to semi-quantitatively evaluate each tangle maturity level. Based on proportions of tangle maturity levels, we classified antibodies as "early" (mostly pretangles and mature tangles), "middling" (mature tangles with pretangles and ghost tangles), and "advanced" (mostly ghost tangles and mature tangles) tangle maturity markers. To summarize tangle maturity predilection, we developed the tangle maturity scale to integrate individual tangle maturity scores. Correlations showed stronger relationships between tangle maturity scale and subsector thickness for more advanced tangle maturity markers in CA1 and subiculum, whereas Braak tangle stage remained consistently correlated throughout markers of the tangle lifespan. To aid in scoring hippocampi, we used machine learning to recognize tangle maturity levels, which performed comparably to a domain expert and showed similar relationships by Spearman correlation. Pattern recognition software was used to assess tangle and neuritic tau burden separately, which generally correlated with Braak stage and neuronal counts. However, tangle-derived tau burden more consistently correlated with hippocampal subsector thickness. In conclusion, we developed manual and automated scoring systems to evaluate tangle maturity levels, demonstrating early 4R, phosphorylated, and oligomeric tau accumulation preceding more advanced 3R and truncated tau. Our study provides supportive evidence of disease-relevant ordering of tau posttranslational modifications in the brain, which may have implications for theragnostic development. These findings underscore the promise of computerized quantitative analyses (i.e., pathomics) for high-throughput feature extraction from whole-slide images to enhance our understanding of microscopically observed morphologic changes.
Keywords: Alzheimer’s disease; artificial intelligence; neurofibrillary tangle maturity; tangle maturity scale; tangle maturity score.
Conflict of interest statement
Conflict of interest MHR, SAL, ZP, JFT, ACW, DMR, RD, CL, NMK, and REK report no disclosures. CMM received grant funding from Eli Lilly and Company. NRG-R has taken part in multicenter trials supported by Eli Lilly, Biogen, Eisai and Cognition Therapeutics which is outside the submitted work. He has received publishing royalties from UpToDate, Inc for a chapter on NPH. DWD is an editorial board member of Acta Neuropathologica, Annals of Neurology, Brain, Brain Pathology, and Neuropathology, and he is editor in chief of American Journal of Neurodegenerative Disease. MEM received grant funding from Eli Lilly and Company and receives consulting fees from Biogen.
Figures
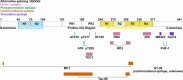





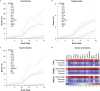
References
-
- Alzheimer A (1911) Über eigenartige Krankheitsfälle des späteren Alters. Zeitschrift für die gesamte Neurologie und Psychiatrie 4: 356–385. 10.1007/bf02866241 - DOI
-
- Alzheimer A (1907) Über eine eigenartige Erkankung der Hirnrinde. Allgemeine Zeitschrift fur Psychiatrie und Psychisch-gerichtliche Medizin 18: 177–179.
-
- Aragao Gomes L, Uytterhoeven V, Lopez-Sanmartin D, Tome SO, Tousseyn T, Vandenberghe R, Vandenbulcke M, von Arnim CAF, Verstreken P, Thal DR (2021) Maturation of neuronal AD-tau pathology involves site-specific phosphorylation of cytoplasmic and synaptic tau preceding conformational change and fibril formation. Acta Neuropathol 141: 173–192. 10.1007/s00401-020-02251-6 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
