This is a preprint.
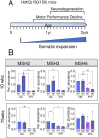
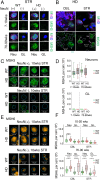
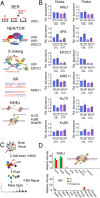
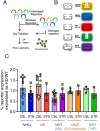
Double strand breaks drive toxicity in Huntington's disease mice with or without somatic expansion
- PMID: 40501854
- PMCID: PMC12154654
- DOI: 10.1101/2025.05.27.654663
Double strand breaks drive toxicity in Huntington's disease mice with or without somatic expansion
Abstract
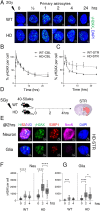
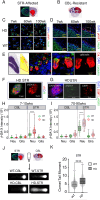
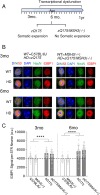
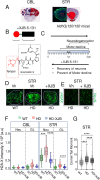
There has been a substantial investment in elucidating the mechanism of expansion in hopes of identifying therapeutic targets for Huntington disease (HD). Although an expanded CAG allele is the causal mutation for HD, there is evidence that somatic expansion may not be the only disease driver. We report here that double strand breaks (DSBs) drive HD toxicity by an independent mechanism from somatic expansion. The mutant HD protein inhibits non-homologous end joining (NHEJ) activity, leading to the accumulation of DSBs. DSBs promote transcriptional pathology in mice that cannot expand their CAG tracts somatically. Conversely, Inhibition of DSBs reverses neuronal toxicity in animals that undergo somatic expansion. Although they coexist in neurons, DSBs and somatic expansion are independent therapeutic targets for HD.
Conflict of interest statement
Competing Interest statement The authors declare no competing interests.
Figures
Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Khan S. et al. The Huntington’s disease drug pipeline: a review of small molecules and their therapeutic targets. Prog Mol Biol Transl Sci. 211,169–207 (2025). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
