This is a preprint.
The specificity and structure of DNA crosslinking by the gut bacterial genotoxin colibactin
- PMID: 40501905
- PMCID: PMC12154741
- DOI: 10.1101/2025.05.26.655968
The specificity and structure of DNA crosslinking by the gut bacterial genotoxin colibactin
Abstract
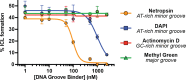
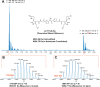
Accumulating evidence has connected the chemically unstable, DNA-damaging gut bacterial natural product colibactin to colorectal cancer, including the identification of mutational signatures that are thought to arise from colibactin-DNA interstrand crosslinks (ICLs). However, we currently lack direct information regarding the structure of this lesion. Here, we combine mass spectrometry and nuclear magnetic resonance spectroscopy to elucidate the specificity and structure of the colibactin-DNA ICL. We find that colibactin alkylates within the minor groove of AT-rich DNA, explaining the origins of mutational signatures. Unexpectedly, we discover that the chemically unstable central motif of colibactin mediates the sequence specificity of crosslinking. By directly elucidating colibactin's interactions with DNA, this work enhances our understanding of the structure and genotoxic mechanisms of this cancer-linked gut bacterial natural product.
Conflict of interest statement
Competing interests: EPB is an inventor on patent applications related to detection and inhibition of colibactin biosynthesis (U.S. Patent 11,617,759, U.S. Patent 12,115,176, U.S. Patent 11,040,951). All other authors declare that they have no competing interests.
Figures




References
-
- El Tekle G., Andreeva N., Garrett W. S., The role of the microbiome in the etiopathogenesis of colon cancer. Annu. Rev. Physiol. 86, 453–478 (2024). - PubMed
-
- White M. T., Sears C. L., The microbial landscape of colorectal cancer. Nat. Rev. Microbiol. 22, 240–254 (2024). - PubMed
-
- Nougayrède J.-P., Homburg S., Taieb F., Boury M., Brzuszkiewicz E., Gottschalk G., Buchrieser C., Hacker J., Dobrindt U., Oswald E., Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851 (2006). - PubMed
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
