This is a preprint.
Rational design of tertiary coordination sphere of a heme-based sensor for two-orders enhanced oxygen affinity
- PMID: 40501969
- PMCID: PMC12154584
- DOI: 10.1101/2025.05.27.656425
Rational design of tertiary coordination sphere of a heme-based sensor for two-orders enhanced oxygen affinity
Abstract
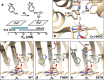
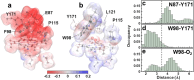
Biological O2 sensing is crucial for diverse physiological functions across all forms of life. Heme-containing proteins achieve this by binding O2 to their iron center and have been found to display O2 affinities spanning several orders of magnitude. Despite decades of investigation into the structure and function of heme-based O2 sensors, the molecular mechanisms that enable the tuning of O2 affinity to match specific physiological roles remain unclear. Here, we utilize the O2 sensing mycobacterial DosS protein as a model system to explore the role of heme iron's tertiary coordination sphere in controlling its O2 affinity. By rationally and systematically modifying the tertiary coordination sphere to promote the formation of a Trp-Tyr-Asn H-bond triad within the heme's distal pocket, we have enhanced the O2 affinity of WT DosS by over 150-fold. The rationally designed DosS exhibited a K d value of 3 ± 1 nM, compared to 460 ± 80 nM for WT DosS. Employing a combination of structural, biochemical, spectroscopic, and computational studies, our analysis of WT and designed DosS variants highlights how the interplay between distal H-bond networks and heme-pocket electrostatics drives large differences in their O2 sensing capabilities. Ultimately, our work shows how metalloenzymes can dramatically alter their sensitivity to diatomic signaling molecules by tuning the tertiary coordination sphere, broadly impacting how we understand related biological sensing and signaling.
Figures


References
-
- Hammarlund E. U., Flashman E., Mohlin S. & Licausi F. Oxygen-sensing mechanisms across eukaryotic kingdoms and their roles in complex multicellularity. Science 370, eaba3512 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
