This is a preprint.
EBSn, a robust synthetic reporter for monitoring ethylene responses in plants
- PMID: 40501970
- PMCID: PMC12154813
- DOI: 10.1101/2025.05.23.655144
EBSn, a robust synthetic reporter for monitoring ethylene responses in plants
Update in
-
EBSn, a Robust Synthetic Reporter for Monitoring Ethylene Responses in Plants.Plant Biotechnol J. 2026 Feb;24(2):698-716. doi: 10.1111/pbi.70302. Epub 2025 Sep 21. Plant Biotechnol J. 2026. PMID: 40976865 Free PMC article.
Abstract
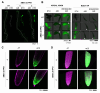
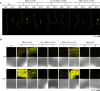
Ethylene is a gaseous plant hormone that controls a wide array of physiologically relevant processes, including plant responses to biotic and abiotic stress, and induces ripening in climacteric fruits. To monitor ethylene in plants, analytical methods, phenotypic assays, gene expression analysis, and transcriptional or translational reporters are typically employed. In the model plant Arabidopsis, two ethylene-sensitive synthetic transcriptional reporters have been described, 5xEBS:GUS and 10×2EBS-S10:GUS. These reporters harbor a different type, arrangement, and number of homotypic cis-elements in their promoters and thus may recruit the ethylene master regulator EIN3 in the context of alternative transcriptional complexes. Accordingly, the patterns of GUS activity in these transgenic lines differ and neither of them encompasses all plant tissues even in the presence of saturating levels of exogenous ethylene. Herein, we set out to develop and test a more sensitive version of the ethylene-inducible promoter that we refer to as EBSnew (abbreviated as EBSn). EBSn leverages a tandem of ten non-identical, natural copies of a novel, dual, everted, 11bp-long EIN3-binding site, 2EBS(-1). We show that in Arabidopsis, EBSn outperforms its predecessors in terms of its ethylene sensitivity, having the capacity to monitor endogenous levels of ethylene and displaying more ubiquitous expression in response to the exogenous hormone. We demonstrate that the EBSn promoter is also functional in tomato, opening new avenues to manipulating ethylene-regulated processes, such as ripening and senescence, in crops.
Conflict of interest statement
Conflict of interest statement The authors declare that they have no competing interests to disclose.
Figures





References
-
- Abeles FB, Morgan PW, Saltveit ME Jr. Ethylene (2nd ed.). In: Abeles FB, Morgan PW, Saltveit ME Jr, editors. Plant Biology, Academic Press, NY: Elsevier Inc. US; 1992. https://shop.elsevier.com/books/ethylene-in-plant-biology/abeles/978-0-0...
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
